Cancer metastasis: breakthrough in therapeutic strategies
Posted: 10 August 2023 | Taylor Mixides (Drug Target Review) | No comments yet
A new discovery from the University of Southern California (USC) on cancer metastasis has opened up new possibilities for combating the spread of this devastating disease.

A breakthrough study conducted by researchers at the Keck School of Medicine of USC has shown a remarkable finding: during moments of stress, cancer cells undergo a transformative process. A crucial protein within these cells migrates to the nucleus, effectively reprogramming them to exhibit increased mobility and invasiveness. This discovery sheds new light on the intricate mechanisms at play within cancer cells when faced with adverse conditions.
Published in the Proceedings of the National Academy of Sciences, a new discovery from the University of Southern California (USC) on cancer metastasis has opened up new possibilities for combating the spread of this devastating disease. Metastasis is the most lethal attribute of cancer cells and clinical decisions regarding treatment are based largely upon the likelihood of developing metastases.1 The research, supported by the National Institutes of Health, centres on a cellular chaperone protein called GRP78, responsible for regulating protein folding within cells. Previous studies led by Dr Amy S. Lee, a prominent biochemistry and molecular medicine professor at the Keck School of Medicine of USC, demonstrated that GRP78 is hijacked under stress conditions, enabling both viral invaders, such as those causing COVID-19, and cancer cells to flourish and resist treatment.
In a serendipitous turn, Dr Lee and her team made an unexpected finding with the potential to shield cells from this hostile takeover. While GRP78 is typically found in a cell compartment known as the endoplasmic reticulum, under stress, it relocates to the cell nucleus, where it profoundly influences gene activities and cell behaviour, enhancing cancer cells’ mobility and invasiveness.
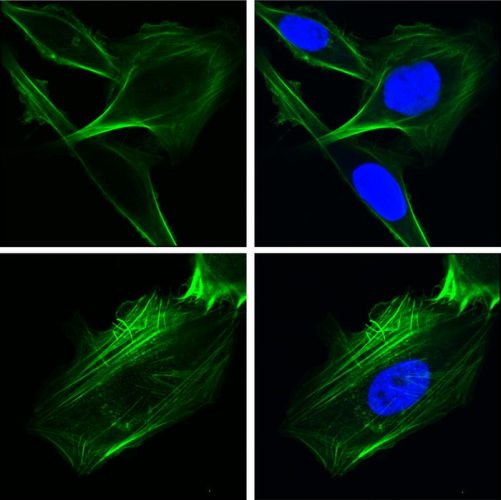

Researchers from the Keck School of Medicine of USC used imaging techniques to study how the protein GRP78 controls cancer cell behavior. In the top row, human lung cancer cells were engineered to over-express GRP78 in the nucleus. In the bottom row, cells lacked GRP78 in the nucleus. The green staining depicts the cytoskeletal protein F-ACTIN which controls cell shape and motility, and the blue staining depicts the nucleus.
The revelation: GRP78 takes centre stage in the nucleus
The research began as an incidental observation when Dr Ze Liu, a postdoctoral researcher in Dr Lee’s lab, was studying how GRP78 regulates the EGFR gene, known for its implication in cancer. Surprisingly, they noticed that GRP78 was controlling EGFR’s gene activity, suggesting that the chaperone protein might have entered the nucleus and adopted a new role. This finding was noteworthy since GRP78 had long been presumed to exist predominantly in the endoplasmic reticulum.
To confirm their hypothesis, the team employed advanced techniques, including confocal microscopy for high-resolution imaging of live cells, to directly observe GRP78’s presence in the nucleus of lung cancer cells and stressed normal cells. Further investigations, using biochemical analysis and mRNA “knock-down” of GRP78, allowed them to identify the signals enabling GRP78’s nuclear entry and to verify its role in stimulating EGFR gene activity.
A twist of fate: GRP78 regulates cell migration and invasion
Continuing their exploration of GRP78’s functions in the cell nucleus, the researchers utilised sophisticated RNA sequencing to compare lung cancer cells engineered to over-express nuclear GRP78 with cells lacking it. Surprisingly, they discovered that the genes regulated by GRP78 in the nucleus were primarily involved in cell migration and invasion, unravelling a previously unknown link between this chaperone protein and cancer cell mobility.
The key interaction was between GRP78 and another cellular protein called ID2, which typically suppresses genes, including EGFR, that are responsible for cell migration. When bound to GRP78, ID2 loses its inhibitory function, leading to increased invasiveness of cancer cells.
Implications for cancer therapeutics and beyond
These findings have wide-ranging implications for cancer treatment and cell biology research. Targeting GPR78 to suppress EGFR in lung cancer or preventing its binding to ID2 could offer promising therapeutic approaches. Additionally, this newfound understanding of GRP78’s migration to the nucleus could inspire further investigations into its role in various cancer types, such as pancreatic, breast, and colon cancer.
Further investigations, using biochemical analysis and mRNA “knock-down” of GRP78, allowed them to identify the signals enabling GRP78’s nuclear entry and to verify its role in stimulating EGFR gene activity.
Moreover, this discovery has the potential to revolutionize the field of cell biology, suggesting that other proteins, typically localized in specific cellular compartments, may also migrate under stress or other triggers, altering cell behaviour in multifaceted ways.
Looking ahead: novel therapeutic avenues
Dr Lee and her team are already exploring drugs that can inhibit GRP78 expression or activity, with ongoing studies indicating that certain small molecules, like YUM70, might even block GRP78’s nuclear activity. These potential therapeutics hold immense promise in halting cancer metastasis and represent a significant step forward in the fight against this devastating disease.
The research at USC has not only shed light on the intricate mechanisms of cancer cell metastasis but has also opened up exciting new avenues for therapeutic intervention. By deciphering the role of GRP78 in the cell nucleus, scientists may soon harness its potential to design more effective and targeted cancer treatments, ultimately offering hope to millions of patients worldwide.
References
- Welch DR. Do we need to redefine a cancer metastasis and staging definitions? [Internet]. IOS Press; 2007 [cited 2023 Jul 26]. Available from: https://content.iospress.com/articles/breast-disease/bd000234
Related topics
Gene Testing, Gene Therapy, Small molecule, Targets
Related conditions
Cancer
Related organisations
Keck School of Medicine of USC, University of Southern California (USC)








