First patient enrolled in fostamatinib trial for sickle cell disease
Posted: 29 January 2025 | Drug Target Review | No comments yet
Rigel Pharmaceuticals has announced the enrolment of the first patient in a Phase I trial evaluating fostamatinib for sickle cell disease.
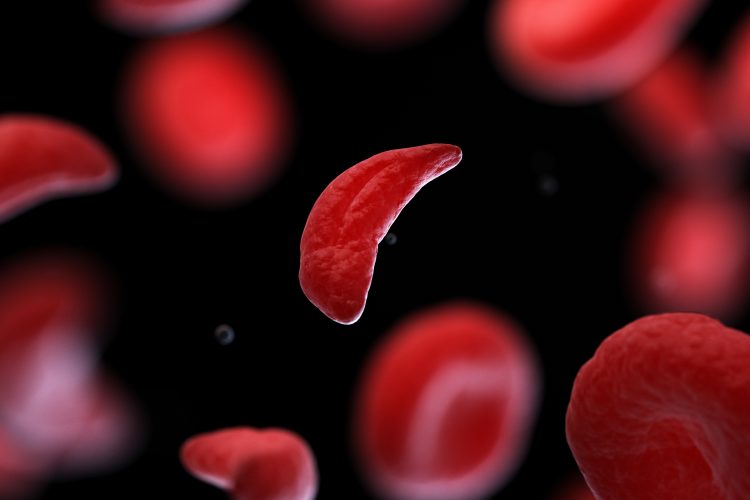

Rigel Pharmaceuticals, Inc. has announced the enrolment of the first patient in a Phase I clinical trial evaluating fostamatinib, the company’s oral spleen tyrosine kinase (SYK) inhibitor, for treating sickle cell disease (SCD). Sponsored by the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH), this study explores the safety and tolerability of fostamatinib in patients suffering from this debilitating condition.
Fostamatinib, marketed as TAVALISSE® in the US for chronic immune thrombocytopenia (ITP), is being studied for its potential to treat complications of sickle cell disease. These complications include severe pain episodes, strokes, and organ dysfunction. Rigel’s collaboration with the NIH/NHLBI marks an important step in exploring new treatment options for a disease that affects more than 100,000 people in the United States and millions worldwide.
Dr Richard Childs, scientific director at NHLBI, noted that preclinical research conducted by the NIH suggests that SYK inhibition, the mechanism of action of fostamatinib, could help reduce complications related to red blood cell sickling and thrombo-inflammation in sickle cell patients. “Our Phase I study evaluating fostamatinib in patients with sickle cell disease is an opportunity to explore a potential new treatment option for a disease that is associated with a high degree of recurrent acute pain events and other acute and chronic potentially life-threatening complications,” said Childs.
Study design and objectives
The Phase I study will enrol approximately 20 patients with sickle cell disease. The trial is led by Dr Swee Lay Thein, senior investigator and chief of the Sickle Cell Branch at NHLBI. Patients will receive escalating doses of fostamatinib, starting at 100 mg twice daily for the first 14 days, with the potential to increase to 150 mg twice daily for an additional 28 days, depending on tolerability.
The primary goal of the study is to evaluate the safety and tolerability of fostamatinib in this patient population. Secondary and exploratory objectives include assessing the drug’s effects on the underlying mechanisms of sickle cell disease, including red blood cell membrane stability, sickling kinetics, and platelet activation. Researchers will also investigate the potential of fostamatinib to impact neutrophil activation and the formation of neutrophil extracellular traps (NETs), which play a key role in the inflammatory processes of sickle cell disease.
The study is being conducted at the NIH Clinical Center in Bethesda, Maryland, with Rigel Pharmaceuticals providing the study material. Dr Raul Rodriguez, president and CEO of Rigel, expressed his excitement about the study, stating, “We are excited to support another important study conducted by the NIH/NHLBI for fostamatinib, as they investigate SYK inhibition and its potential to benefit patients with sickle cell disease.”
Looking ahead
While the Phase I study is still in its early stages, the collaboration between Rigel Pharmaceuticals and NIH/NHLBI offers hope for a new treatment avenue for sickle cell disease. If successful, this trial could pave the way for future studies that explore the broader potential of SYK inhibitors in haematologic and other inflammatory disorders.
Related topics
Biopharmaceuticals, Clinical Trials, Drug Discovery, Drug Discovery Processes, Translational Science
Related conditions
Sickle cell disease (SCD)
Related organisations
National Heart Lung and Blood Institute (NHLBI), National Institutes of Health (NIH), Rigel Pharmaceuticals
Related people
Dr Raul Rodriguez, Dr Richard Childs, Dr Swee Lay Thein