Navigating the AI revolution: a roadmap for pharma’s future
Posted: 13 March 2025 | Alessio Zoccoli, Carlos N Velez, Remco Jan Geukes Foppen, Vincenzo Gioia | No comments yet
AI-driven drug development, powered by advanced models and expanding data access, is becoming a reality. Learn why navigating regulatory hurdles and mastering biology’s inherent complexities are crucial to fully unlocking its potential.

Planning the journey from data to deliverables
The future of AI-enabled drug development benefits from the continued advancement of multimodality and clinical genomics, with a focus on integration, efficiency and personalisation to transform both care and R&D. These evolving technologies have enormous potential to overcome current limitations in the field. Advanced AI models will handle complex datasets, improving the understanding of drug-target interactions. Applying explainable AI (xAI) tools to small language models enhances transparency, reliability and accuracy of outputs, which can significantly facilitate regulatory acceptance. The operational impact of xAI is substantial, especially given that 37 percent of the market views the explanation of results from GenAI algorithms as a strategic priority that goes beyond regulatory compliance. AI adoption will grow, but will require robust infrastructure, pipeline standardisation and training. The journey from distrust to collaboration among industry, academia and regulators will expedite integration, minimise clinical failures and enhance economic sustainability.
The landscape of data augmentation remains dynamic, with ongoing discussions about optimal data access policies and sharing strategies. Balancing regulatory policy (eg, US Food and Drug Administration (FDA) and European Medicines Agency (EMA)) with patient privacy and commercial confidentiality remains crucial. Yet Ilya Sutskever, OpenAI at NeurIPS, argues that data augmentation has limits: “Data is not growing because we only have one internet. You could even say data is the fossil fuel of AI. It was created in a certain way and now we are using it. We have reached peak data, and there will be no more – we must work with what we have”. The drug and healthcare sectors, however, offer a fertile ground for AI adoption, driven by a continuous influx of new data sources.
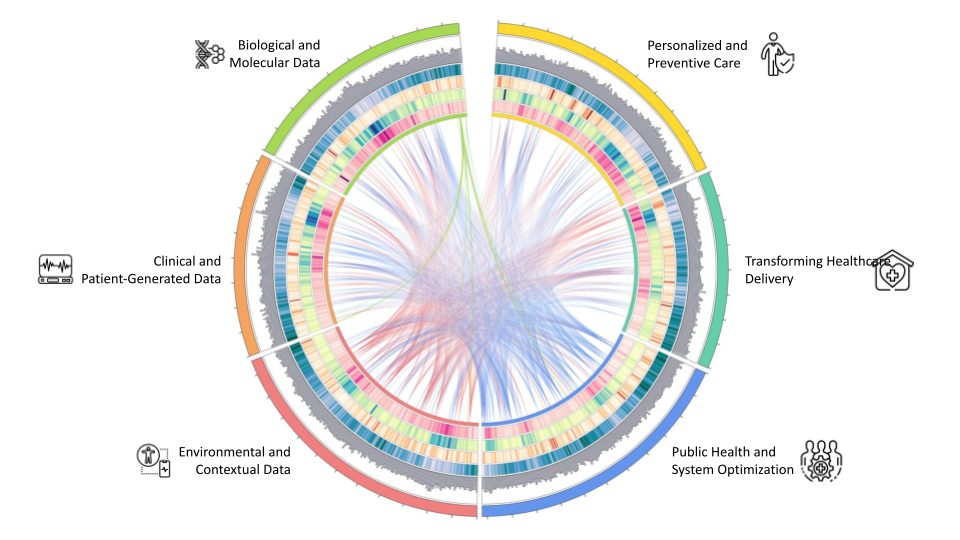
Figure 1: Graphical representation of disparate data sources and data modalities that can unlock the full potential of AI to transform drug development.
Data generation for drug discovery extends beyond the internet, encompassing data from laboratory instruments, empathic increase in radiomic output, and real-world evidence (RWE) from clinical settings (Figure 1). Notably, US electronic health record data is estimated at 150 petabytes, with only five percent currently utilised. Significant efforts are underway to unlock the vast potential of these electronic health records. Elnora AI exemplifies an innovative data augmentation strategy by collecting ‘negative data’ directly from academic researchers, focusing on detailed lab protocols. Negative data is often much larger in size than published data because it includes raw data from failed experiments, clinical trials with no significant effect and studies with null results. AI-powered digital twins are another source of data augmentation without the common restrictions of patient privacy. Surveys (eg, 2024 State of Tech in Biopharma Report) predict a doubling of R&D data year-over-year in 2025, highlighting the continued growth of available data. With relatively equal access to technologies, digital strategies may still diverge. As more digital and data capabilities come online, how will investment priorities shift?
A data-driven approach to higher success rates
Choosing between long lead times, high costs and a low probability of success (PoS), the most valuable lever in drug development is PoS. Without compelling evidence of AI delivering a higher PoS, improving speed and reducing cost at least shows there is a path to getting to higher quality drugs faster and cheaper. This path appears to be driven by data augmentation, folding into clinical genomics and multimodal approaches, which together hold promise for accelerating development and improving outcomes. Techbio companies have initiated this approach. Now even non-AI native big pharma companies take a similar view on using clinical genomics with integrated multimodality.
The synergy between regulatory, technological and methodological changes has had a positive impact on the entire drug development cycle. With a more accurate selection of targets and better stratification of patients, many failures that previously occurred in the clinical phases can be prevented. The development of targeted drugs for genetically defined patient subgroups has significantly improved the efficacy of treatments, while reducing the risks of adverse effects.
A critical aspect is the balance between the speed of development and the quality of drug candidates for testing. While AI can significantly accelerate the discovery process, it is essential that speed does not compromise the robustness of the data and the likelihood of clinical success, so that effective treatments can ultimately reach the patients. The key question then becomes not only how quickly a new drug can be developed, but also how much additional time will be invested in optimising AI models to improve the likelihood of success in the clinical phases.
Data-driven AI is improving the development of drugs. Expanding data access, coupled with advanced models and technologies like multimodality and clinical genomics, enables more precise target selection, patient stratification, and personalised treatments, promising higher success rates and greater returns on investment. This is an AI generated image.
Despite these advances, significant challenges remain. These include managing biological complexity, difficulties in data integration and regulatory requirements. The increasing complexity of target molecules and the exploration of advanced therapeutic modalities, such as bispecific antibodies and cell therapies, require continuous refinement of AI algorithms and models. However, the current landscape highlights how the combination of AI, multimodality and clinical genomics represents not only a promise for the future, but a reality that is already redefining the paradigms of drug discovery and development.
Although AI models have shown great potential, their generalisation capacity is still limited. The dependence on high-quality and balanced training data can introduce biases in the models, which reduces the reliability of the results, especially when treating rare diseases or poorly studied targets. Furthermore, the explainability of AI predictions remains an unresolved issue, making it difficult for regulatory authorities to adopt these technologies.
Current regulatory agencies, such as the FDA, have not yet authorised any large language models (LLMs) in medicine and are not fully adapted to AI-driven and multimodal data-driven approaches in drug discovery and development. The lack of standardised guidelines for the validation and approval of AI-driven pipelines represents a significant obstacle, especially for models that use sensitive genomic and clinical data. Furthermore, the difficulty of demonstrating the robustness and reliability of AI predictions at different clinical stages may slow down the approval of new drugs.
Distinguishing drugs solely by their development method (AI vs. traditional) is increasingly challenging. It is a continuum that will evolve as technology progresses. Drug discovery is inherently complex and time-consuming, often requiring over a decade to advance a sufficient number of candidates for rigorous analysis. This presents a significant hurdle for investors, as reliable benchmarks for assessing investment opportunities may be limited. Investors should define a decision matrix for funding companies with access to data, with robust and validated AI platforms, led by experienced teams with a proven track record in drug discovery or related fields, and maintain a diversified portfolio to mitigate risk. Longitudinal outcome tracking is crucial for validating the efficacy of AI-driven drug discovery and determining its strategic role in addressing future challenges within the field.
This article, along with other insightful analyses, will be featured in our upcoming report, Beyond the Lab – Artificial Intelligence, launching on 21st March 2025. Be sure to download the report to explore additional groundbreaking advancements in AI-driven drug development and discover how artificial intelligence continues to shape the future of healthcare innovation.
About the authors

Remco Jan Geukes Foppen, PhD, is an AI and life sciences expert specialising in the pharmaceutical sector. With a global perspective, he integrates and implements AI-driven strategies that impact business decisions, always considering the human element. His leadership has driven international commercial success in areas including image analysis, data management, bioinformatics, advanced clinical trial data analysis leveraging machine learning and federated learning. Remco Jan Geukes Foppen’s academic background includes a PhD in biology and a master’s degree in chemistry, both from the University of Amsterdam.
 Vincenzo Gioia is an AI innovation strategist and a business and technology executive, with a 20-year focus on quality and precision for the commercialisation of innovative tools. Vincenzo specialises in artificial intelligence applied to image analysis, business intelligence and excellence. His focus on the human element of technology applications has led to high rates of solution implementation. He holds a master’s degree from University of Salerno in political sciences and marketing.
Vincenzo Gioia is an AI innovation strategist and a business and technology executive, with a 20-year focus on quality and precision for the commercialisation of innovative tools. Vincenzo specialises in artificial intelligence applied to image analysis, business intelligence and excellence. His focus on the human element of technology applications has led to high rates of solution implementation. He holds a master’s degree from University of Salerno in political sciences and marketing.
 Alessio Zoccoli applies AI for a sustainable future. His deep understanding of industry applications and technical expertise drives innovation in AI-powered solutions for complex business challenges. He specialises in cutting-edge advancements in natural language processing, computer vision, and generative AI. He is a senior data scientist and holds a master’s degree from Roma Tre University in computer engineering, where he also held the role of research fellow.
Alessio Zoccoli applies AI for a sustainable future. His deep understanding of industry applications and technical expertise drives innovation in AI-powered solutions for complex business challenges. He specialises in cutting-edge advancements in natural language processing, computer vision, and generative AI. He is a senior data scientist and holds a master’s degree from Roma Tre University in computer engineering, where he also held the role of research fellow.
 Carlos N Velez, PhD, MBA, is a pharmaceutical and biotechnology strategic advisor, with 25 years’ experience in consulting, venture capital, corporate strategy and entrepreneurship. Carlos specialises in helping pharmaceutical and biotechnology companies develop their in- and out-licensing strategies, with additional expertise and experience in portfolio assessment and prioritisation, drug candidate valuation, valuation and related services. He also develops and presents customised training programmes (both live and virtual) for companies seeking to improve their in- and out-licensing processes. He holds a PhD in pharmacy from the University of North Carolina at Chapel Hill, and an MBA from the Rochester Institute of Technology.
Carlos N Velez, PhD, MBA, is a pharmaceutical and biotechnology strategic advisor, with 25 years’ experience in consulting, venture capital, corporate strategy and entrepreneurship. Carlos specialises in helping pharmaceutical and biotechnology companies develop their in- and out-licensing strategies, with additional expertise and experience in portfolio assessment and prioritisation, drug candidate valuation, valuation and related services. He also develops and presents customised training programmes (both live and virtual) for companies seeking to improve their in- and out-licensing processes. He holds a PhD in pharmacy from the University of North Carolina at Chapel Hill, and an MBA from the Rochester Institute of Technology.
Literature
- “Explainambiguity:” When What You Think Is Not What You Get [Internet]. Lifescienceleader.com. 2024 [cited 2025 Feb 7]. Available from: https://www.lifescienceleader.com/doc/explainambiguity-when-what-you-think-is-not-what-you-get-0001
- Gioia V, Geukes Foppen RJ. Correct But Misleading: AI Hallucinations In Complex Decision-Making [Internet]. com. 2024 [cited 2025 Feb 7]. Available from: https://www.lifescienceleader.com/doc/correct-but-misleading-ai-hallucinations-in-complex-decision-making-0001
- Geukes Foppen RJ, Gioia V, Zoccoli A. Scienze della vita: la svolta degli Small Language Model [Internet]. Agenda Digitale. 2024 [cited 2025 Feb 7]. Available from: https://www.agendadigitale.eu/sanita/scienze-della-vita-la-svolta-degli-small-language-model/
- Haslam C. How AI will reshape pharma in 2025 [Internet]. Drug Target Review. 2024. Available from: https://www.drugtargetreview.com/article/154981/how-ai-will-reshape-pharma-by-2025/
- Mahon P, Hall G, Dekker A, et al. Harnessing oncology real-world data with AI. Nature Cancer. 2023 Dec 15;4(12):1627–9.
- 2023 State of Tech in Biopharma Report [Internet]. www.benchling.com. Available from: https://www.benchling.com/state-of-tech
- Geukes Foppen RJ, Gioia V, Velez CN. AI, PoS, And ROI: An Alphabet Soup Of 21st Century Drug Development [Internet]. Lifescienceleader.com. 2024. Available from: https://www.lifescienceleader.com/doc/ai-pos-and-roi-an-alphabet-soup-of-st-century-drug-development-0002
- Geukes Foppen RJ, Gioia V, Velez CN. AI, PoS, And ROI: An Alphabet Soup Of 21st Century Drug Development [Internet]. Lifescienceleader.com. 2024 [cited 2025 Feb 7]. Available from: https://www.lifescienceleader.com/doc/ai-pos-and-roi-an-alphabet-soup-of-st-century-drug-development-0001
- Warraich HJ, Tazbaz T, Califf RM. FDA Perspective on the Regulation of Artificial Intelligence in Health Care and Biomedicine. JAMA [Internet]. 2024 Oct 15; Available from: https://jamanetwork.com/journals/jama/fullarticle/2825146
- Office. FDA Proposes Framework to Advance Credibility of AI Models Used for Drug and Biological Product Submissions [Internet]. U.S. Food and Drug Administration. 2025. Available from: https://www.fda.gov/news-events/press-announcements/fda-proposes-framework-advance-credibility-ai-models-used-drug-and-biological-product-submissions
- Geukes Foppen RJ, Gioia V, Gupta S, et al. Methodology for Safe and Secure AI in Diabetes Management. Journal of Diabetes Science and Technology [Internet]. 2024 Dec 26; Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC11672366/
- Geukes Foppen RJ, Gioia V, Zoccoli A, Velez CN. Using clinical genomics and AI in drug development to elevate success. (2025). Drug Target Review February Edition https://www.drugtargetreview.com/article/155906/clinical-genomics-ai-drug-success/