New advances in hormone replacement therapy set to transform care
Posted: 26 March 2025 | Dr Charles P. Virden – CEO of VitalTE, Dr Lisa Stehno-Bittel – President and Founder of Likarda | No comments yet
Hormone replacement therapy has long been used to treat low hormone levels, but existing delivery methods struggle with poor adherence and inconsistent hormone delivery. Lisa Stehno-Bittel of Likarda and Charles Virden of VitalTE discuss a novel approach using hormone-filled microbeads in hydrogels for steady, extended release after monthly self-injections.

Hormone replacement therapy (HRT) for low hormone levels is one of the oldest treatments utilised in Western medicine and can be traced back centuries. Commercial hormone distribution in the US began as early as 1917, when thyroxine was introduced to treat goiter,1 soon followed by insulin to treat type 1 diabetes.2
Sex hormone replacement also gained in popularity, notably starting in 1940, when conjugated equine oestrogen was approved for the treatment of postmenopausal women.3 The scalable production of oestrogen led to its popularity and by the 1980-1990s, oestrogen (Premarin) was named the number one drug prescribed in the US.
The story is similar for testosterone replacement, which was first used to treat the symptoms of hypogonadism in the early 20th century. In 1935, testosterone was chemically synthesised, representing a major milestone in the field.4 Shortly after, testosterone pellets hit the market, followed by injectable ester forms. Today, testosterone is still used for hypogonadism, as well as for sexual dysfunction5 and delayed puberty.6 Both testosterone and oestrogen are also used for gender-affirming therapy.7
There have been few significant advancements in the administration of testosterone or oestrogen in over 20 years, and many approaches have not changed dramatically in 70 years. It is unsurprising, therefore, that only 20 percent of patients with deficiencies in sex hormone levels are being treated, creating demand from both physicians and patients for improved testosterone and oestrogen preparations for clinical use.
Hormone replacement requires treatment for the rest of a person’s life – thus, the greater the patient burden and number of side effects with a given drug, the lesser the compliance.
Patients require therapy options with the following characteristics:
- Can be self-administered
- Extended release requiring infrequent administration
- Provides a steady basal release of hormone that achieves levels within normal ranges
- Has a stable shelf life
- Is reasonably priced
- Lacks side effects.
Sex hormone replacement options
Despite previous safety concerns, recent data have demonstrated that HRT is safe and beneficial.[viii] As a category, the replacement of sex hormones from multiple sources, including animals and bacteria, have been shown to be safe and effective.3 Yet, there remain limitations in the administration of hormones, typically owing to their short half-life.
Table 1 summarises the different administration approaches for HRT, citing the year they were first approved by the US Food and Drug Administration (FDA), along with the advantages and disadvantages of each preparation. It is clear from the table that patients have a variety of choices in the method of administration. Still, there are major limitations for each offering, and a lack of new technological advancements in the field. This has resulted in poor patient compliance for HRT.
Table 1: History of hormone administration methods
|
Administration |
Product |
Year approved |
Advantages |
Disadvantages |
Ref |
|
Crème |
Premarin |
1942 |
Self-application, can rub off before being absorbed. Not as readily absorbed with peak at 5-8 hours. |
Allergic reaction, treatment limited to vaginal symptoms, can increase risk of endometrial cancer |
9 |
|
Oral |
Enovid |
1960 |
Ease of self-application. Safer compared to creams or patches |
Half-life of less than 24 hours |
10 |
|
Pellets
|
TestoPel |
1972
|
3-4 applications/ year, better compliance, consistent T blood levels |
Invasive implantation that requires clinic visit, possible extrusions, limits activity after implantation |
11 |
|
Intramuscular injection |
Depo-Testosterone |
1979 |
Less frequent dosing than topical applications |
Peaks and troughs, painful, requires daily or bi-weekly injections causing fluctuations in mood/libido |
4, 12 |
|
Patches |
Androderm |
1995 |
Safer than oral preparations, consistent low dose, avoids first-pass through liver |
Dosing inconsistent, can cause skin irritation. Cannot use the same patch site for 7 days, due to blistering |
9, 13 |
|
Gel |
AndroGel |
2003 |
Ease of use, non-invasive, less skin irritation compared to patches. |
Daily applications, erratic absorption, low long-term compliance, risk of transfer to others, expense |
14 |
Due to the lack of acceptable options for patients, VitalTE and Likarda joined forces with the goal of developing new HRTs to be administered by the patient with a monthly subcutaneous injection, resulting in the slow release of testosterone or oestrogen. This unique approach aims to utilise novel hydrogels to stabilise the hormone, allowing steady release of an active ingredient when injected into the body.
Hydrogels as delivery systems
Hydrogels are three-dimensional networks of aqueous polymers that have been used in biotechnology products, specifically medical devices, since the 1960s. Alginate, the polymer most frequently used in medical applications, can however cause severe immune reactions.15 Biocompatible alternatives are available, but manufacturing methods to form injectable microbeads were lacking or expensive and complex to scale. They utilised either oil emulsion, which introduces a non-biocompatible oil into the manufacturing process, or else they required complex microfluidics technology that is limited in scale. A new manufacturing method was clearly required.
Core Shell Spherification (CSS) is an encapsulation process that represents a unique approach to creating injectable hydrogel microspheres for slow release of the active ingredient. It is fully aqueous, thus avoiding the oil in emulsion techniques, and is based on a scalable spray technology. Likarda initially developed CSS to overcome the lack of effective delivery systems for cell therapies. It subsequently demonstrated promise for the administration of peptides, monoclonal antibodies and small molecules.
Table 2 compares the characteristics of the microbead hormones with the other administration methods. Crucially, the only extended-release delivery system commercially available today utilises pellets and requires surgical implantation. By instead formulating hormones in biocompatible hydrogel microbeads, the high concentration of hormone needed for extended release is not immediately exposed to the surrounding tissue, thus reducing the likelihood of localised irritation or off-target effects. As the hydrogel degrades in the body, the hormone slowly diffuses to be taken up by the bloodstream.
Table 2: Comparison of subcutaneous hydrogel administration
|
Method of Administration |
Microbead SubQ injections |
Pellet |
Oral |
Patch |
Gel |
IM injections |
|
Self-Administration |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
Extended Release |
Yes |
Yes |
No |
No |
No |
No |
|
Effective |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Stable |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Lacks side effects |
To be determined |
No |
No |
No |
No |
No |
Proteins can be stabilised by linking them to a hydrogel, but many factors determine the ability of a hydrogel to release an active ingredient within the target time and at the target concentration. Those factors include the molecular weight of the starting polymer, the polymer chemistry, the size of the microbeads, and the location in which they are implanted. Early data demonstrates that the half-life of a peptide can be extended from 12 hours in solution to 10 days or longer in specific hydrogels.
Hormone-containing microbeads are effectively micropellets – similar to oestrogen or testosterone pellets in that they are compacted active ingredient, but injectable. Early pre-clinical studies of CSS-encapsulated hormones show a steady release over time.
Figure 1 provides a scanning electron micrograph of a testosterone-laden microbead, which is entirely infused with testosterone. Without the testosterone, the hydrogel polymer has little mass and shrinks flat when prepared for scanning electron microscopy.
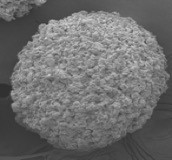

Figure 1: Scanning electron microscopy image of testosterone-filled microsphere.
Further, the hydrogel polymer used in development by VitalTE is hyaluronic acid, a compound that has been used for medical purposes since 1968.16 Currently, it forms the basis of both dermal fillers and joint supplements. The safety profiles of hyaluronic acid, oestrogen and testosterone are all well-known. Encapsulating compacted hormone with hyaluronic acid results in an improved delivery system comprised of well-characterised materials. While the first iteration of the product is aimed at a monthly injection, it is possible to alter the crosslinkers of the hydrogel to produce longer-lasting versions.
Conclusion
After years of suboptimal methods for HRT, a novel combination of hydrogel and hormone may achieve a potential therapy that can finally meet the needs of patients, providing consistent levels of hormone replacement that mitigate symptoms, alongside the convenience of self-administration and infrequent dosing. Further, the combination of hydrogel microbeads and hormone therapies are unlimited, including for molecules like insulin.
References
[1] McAninch E, Bianco A. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164(1):50-6.
[2] Flier J. Starvation in the midst of plenty: reflections on the history and biology of insulin and leptin. Endocr Rev. 2018;40(1):1-16.
[3] Cho L, Kauntiz A, Faubion S, et al. Rethinking menopausal hormone therapy: form whom, what, when and how long? Circulation. 2023;147(7):597-610.
[4] Neischlag E, Neischlag S. Testosterone deficiency: a historical perspective. Asian J Andrology. 2014;16:161-8.
[5] ACP issues guideline for testosterone treatment in adult men with age-related low testosterone | ACP Newsroom | ACP [Internet]. www.acponline.org. American College of Physicians; 2020 [cited 2025 Jan 17]. Available from: https://www.acponline.org/acp-newsroom/acp-issues-guideline-for-testosterone-treatment-in-adult-men-with-age-related-low-testosterone
[6] Vogiatzi M, Tursi JP, Jaffe JS, et al. Testosterone Use in Adolescent Males: Current Practice and Unmet Needs. J Endocr Soc. 2020 Oct 30;5(1):bvaa161. doi: 10.1210/jendso/bvaa161. PMID: 33294762; PMCID: PMC7705876.
[7] Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016 Dec;5(6):877-884. doi: 10.21037/tau.2016.09.04. PMID: 28078219; PMCID: PMC5182227.
[8] Cagnacci A, Venier M. The controversial history of hormone replacement therapy. Medicina. 2019;55(9):602.
[9] Kohn G, Rodriguez K, Pastuszak A. The history of estrogen therapy. Sex Med Rev. 2020;7(3):416-21.
[10] Stefanick M. Estrogens and progestins: background and history, trends in use and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(12):64-73.
[11] McCullough A. A review of testosterone pellets in the treatment of hypogonadism. Curr Sex Health Rep. 2014;6(4):265-9.
[12] Shoskes J, Wilson M, Spinner M. Pharmacology of testosterone replacement therapy preparations. Trnasl Androl Urol. 2016;5(6):834-43.
[13] Gurayah A, Dullea A, Weber A, et al. Long vs short acting testosterone treatments: a look at the risks. Urology. 2024;172:5-12.
[14] Madsen M, den Heiger M, Pees C, et al. Testoerone in men with hypogonadism and transgender males: a systematic review comparign three different preparatiosn. Endocr Connect. 2022;11(8):e220112.
[15] Keown A. Sigilon uncovers possible reason behind haemophilia A trial hold. News [Internet]. 2021.
[16] Price R, Berry M, Navsaria H. Hyaluronic acid: the scientific and clinical evidence. J Plastic, Recon, Aesthetic, Surgery. 2007;60(10):1110-9.
About the authors


Dr Lisa Stehno-Bittel is President and Founder of Likarda and Professor Emeritus at University of Kansas Medical Center. Lisa is a fellow in the American Institute of Medical and Biological Engineering. She has a background in cell biology, biophysics and pharmacology and was a professor and Chair at the University of Kansas for over 20 years. She has numerous awards including Ingram Magazine’s “50 Kansans You Should Know”, the 2018 Kansas Entrepreneur of the Year Award and the Marjorie S. Sirridge Excellence in Medicine and Science Award.


Dr Charles P Virden, MD, is double-boarded in plastic and reconstructive surgery and anti-ageing medicine. In 1987, he graduated from the University of Southern California with a medical degree and completed his general studies internship and residency at the University of California, San Diego. He completed a research fellowship and his residency in plastic surgery. Virden moved to Reno, NV, in 1995 to open his private practice. Since 2015, Virden has served as a volunteer clinical professor at University of California, San Diego (UCSD) Division of Plastic Surgery. He is a fellow of the American College of Surgeons and lead author on multiple research publications concerning the diagnosis and care of dermal disorders and reconstructive surgery. He is Founder and CEO of VitalTE as well as Medical Director and Owner of TheraPellET, where he developed his patented Virden Method™ for reducing procedural trauma.
Related topics
Drug Delivery, Hormones, Pharmacology, Regenerative Medicine
Related conditions
Goiter, Hypogonadism, type 1 diabetes