Nanoparticle therapy shows potential to prevent spread of pancreatic cancer to liver
Posted: 21 March 2025 | Jon Shepheard - Advisory Editor | No comments yet
Pancreatic cancer is notoriously hard to treat, especially when it spreads to the liver in advanced stages. Researchers at UCLA’s California NanoSystems Institute (CNSI) have developed an innovative nanoparticle technology to tackle this challenge.

Pancreatic cancer remains one of the most challenging and aggressive forms of cancer, characterised by a poor prognosis and high fatality rates. A significant obstacle in treating this disease is its tendency to be diagnosed at an advanced stage, by which time it has often metastasised, often to the liver. Nearly half of all patients diagnosed with pancreatic cancer will develop liver metastases, further complicating an already difficult treatment landscape.
However, a team of researchers at the California NanoSystems Institute at UCLA (CNSI) has developed a promising new technology aimed at addressing this challenge head-on. Their innovation focuses on reprogramming the liver’s immune system to target and destroy pancreatic cancer cells that have metastasised to the liver.
The liver’s hidden role in cancer progression
Under normal conditions, the liver plays a crucial role in processing substances from the gut, including food and other foreign compounds. To prevent overreaction by the immune system, the liver must suppress immune responses to harmless substances. However, this immunosuppressive environment also enables cancer cells to evade immune detection, creating an ideal setting for tumour growth.
The researchers at CNSI recognised this dynamic and sought to find a way to counteract the liver’s immune tolerance, aiming to reprogram it to fight cancer rather than enable its growth. Their solution involves a liver-targeting nanoparticle designed to deliver a combination of an mRNA vaccine and a small molecule that enhances the immune response.
The nanoparticle solution
This nanoparticle, which is just a billionth of a meter in size, works by delivering two key components to the liver’s immune cells: an mRNA vaccine that targets a specific marker found in pancreatic cancer and a small molecule designed to boost immune activity. The mRNA in the vaccine encodes instructions to activate an immune response against a mutated protein frequently found in pancreatic cancer cells – KRAS.
This immunosuppressive environment also allows cancer cells to evade immune detection, creating a favourable niche for tumour growth
KRAS is a gene that plays a crucial role in regulating cell growth, and when mutated, it can drive the development and spread of cancer. In their study, published in ACS Nano, the researchers demonstrated that the nanoparticle successfully targeted these KRAS mutations, leading to the activation of the immune system’s killer T cells. These cells specifically targeted and killed the cancer cells, reducing the size and spread of pancreatic cancer metastases in the liver.
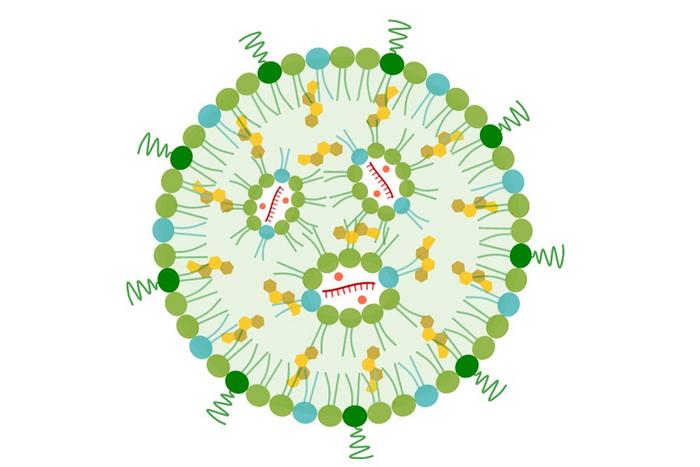

A UCLA-developed nanoparticle is aiming to reprogram the liver in humans to attack pancreatic cancer with an mRNA vaccine (curved, hatched red lines) and an immune-activating fragment of genetic material (red dots). Credit: UCLA
Promising results in lab models
In experiments using animal models, the nanoparticle treatment proved to be highly effective. Mice with pancreatic cancer that had spread to the liver and were treated with the nanoparticles showed significantly smaller and less-dispersed tumours compared to untreated mice. These treated mice also lived longer, demonstrating the potential of this technology to not only treat existing metastases but to prevent further cancer progression.
In experiments using animal models, the nanoparticle treatment proved to be highly effective
One of the most exciting findings from this study was the generation of immune memory cells. These cells, which ‘remember’ the tumour antigens, could provide long-term protection against cancer recurrence. In fact, when blood from treated mice was transferred to untreated mice, the recipients survived significantly longer than those who had not received the transfusion, suggesting that the treatment may have the potential to act as a vaccine for pancreatic cancer.
A personalised approach to cancer treatment
Dr André Nel, a distinguished professor of medicine at UCLA and the corresponding author of the study, envisions the nanoparticle technology as a platform for personalised cancer treatment. By using comprehensive genetic testing, oncologists could identify specific tumour mutations, such as KRAS, in a patient’s cancer cells. This information could be used to customise the nanoparticle’s contents, tailoring the therapy to each individual’s unique cancer profile for maximum efficacy.
Beyond pancreatic cancer, this technology holds promise for other cancers that frequently metastasise to the liver, such as breast and lung cancer. The ability to target cancer cells based on their specific genetic mutations could revolutionise cancer treatment across multiple types of tumours.
Addressing safety concerns
One of the key challenges in cancer therapy is managing toxicity, especially when activating powerful immune pathways. The Stimulator of Interferon Genes (STING) pathway, which the nanoparticle activates, can sometimes lead to harmful systemic inflammation. However, the researchers noted that in their study, no toxicity was observed. They attribute this safety to the localised activity of the STING pathway within the liver, preventing the widespread inflammation that might occur with other immune-stimulating treatments.
Future directions
This novel nanoparticle therapy is still in the early stages, but its potential applications are vast. The researchers are already working on extending the technology to other types of cancers that commonly spread to the liver. The team are also investigating ways to target nanoparticles to other immune organs, such as the spleen, to strengthen the body’s natural defences against tumours. Additionally, they are exploring the combination of this nanoparticle technology with immune-stimulating chemotherapy, a dual approach that could offer a powerful strategy against pancreatic and other hard-to-treat cancers.
This study was published in ACS Nano.
Related topics
Bioengineering, Cancer research, Immunotherapy, Nanomedicine, Nanoparticles, Nanotechnology, Oncology
Related organisations
California NanoSystems Institute at UCLA (CNSI)
Related people
Dr André Nel