The rising impact of biomarkers in early clinical development
Posted: 7 April 2025 | Dr Cyril Clarke (Vice President at ICON Biotech) | No comments yet
Dr Cyril Clarke at ICON Biotech reveals how biomarkers are transforming early-phase clinical trials by offering valuable insights into the safety and efficacy of novel therapies.


As our understanding of the underlying biology of disease grows more sophisticated, emerging therapies operate on increasingly complex biopathological systems and mechanisms. Advanced strategies and tools are being implemented to establish the safety and efficacy of new therapeutic modalities, with the development of new biomarkers becoming one of the most relevant approaches for enhancing the precision and utility of early-stage studies.
A surrogate endpoint is a marker used in clinical trials as a substitute for a direct clinical outcome. Instead of measuring the actual outcome (such as survival), surrogate endpoints track a biomarker or other indicator that is thought to be predictive of the real outcome. Many biomarkers have proven effective as surrogate endpoints. Haemoglobin A1c (HbA1c) is a validated surrogate endpoint for the reduction of microvascular complications associated with diabetes mellitus; reduced HIV-RNA levels serve as an endpoint for HIV disease control; and a reduction in low-density lipoprotein (LDL) cholesterol is used as an endpoint indicating lower likelihood of cardiovascular events.1
Biomarkers are rapidly advancing in areas such as transcriptomics, proteomics and epigenetic profiling. These fields explore highly precise biological processes related to RNA, specific proteins and gene expression mechanisms. For example, transcriptomic processes are showing the potential to identify and track failures in gene expression and gene regulation of amyloid and tau-related biomarkers, understood as precursors to the onset of Alzheimer’s disease (AD).2 This progress has implications for diagnosis, therapeutic efficacy, and potentially establishing clinically relevant endpoints.
The roles and applications of biomarkers
In 2001, the National Institutes of Health Biomarkers Definitions Working Group provided an early definition of biomarkers as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”4 The definition has since evolved and expanded to include molecular signatures of pharmacodynamics and therapeutic response.
There are several types of biomarkers to consider. Safety biomarkers account for adverse effects of a therapy under study. Susceptibility or risk biomarkers can detect the likelihood of a patient developing a disease or medical condition, which is crucial for treatments that are most effective before the onset of symptoms. Diagnostic biomarkers typically confirm or establish a diagnosis and are often used in selecting patient populations for clinical trials. Monitoring biomarkers can help assess changes in a disease, its level of expression, or the extent of its progression. . Prognostic biomarkers can help determine the likelihood of a clinical event, disease recurrence or progression, which is especially useful for enriching studies with patients more likely to experience a relevant event, thereby improving a trial’s statistical power.
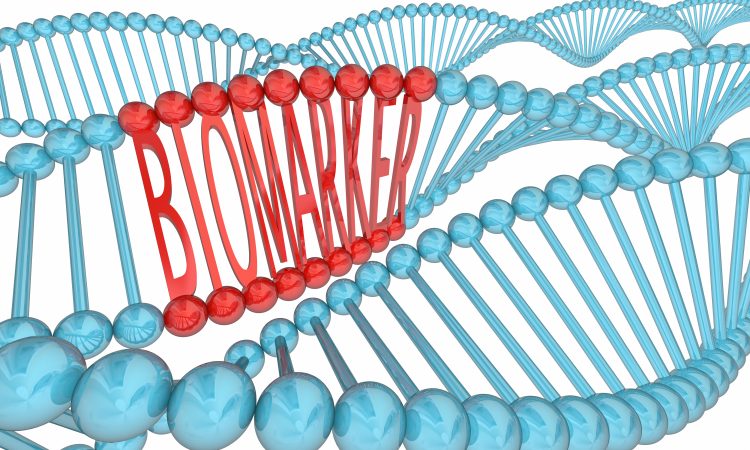

A biomarker is a measurable indicator of a biological process, disease state, or response to a treatment. They are valuable in improving diagnosis, monitoring disease progression, predicting treatment outcomes, and guiding more precise and effective therapies.
Mechanism-driven biomarkers can introduce crucial in-human data about the biological processes involved in a disease or its treatment, as well as essential safety data. Combined with preclinical data, this can comprise a body of evidence that proves crucial to later pivotal trials. Early phase trials can potentially use exploratory biomarker-driven endpoints that provide simultaneous insights into the pharmacodynamics and efficacy of a treatment. Investigational products with novel mechanisms of action are also assessed for safety in unique ways, creating complexities that can be more dynamically and effectively monitored using biologically relevant biomarkers. In a recent survey conducted by ICON, Plc, biomarker selection was identified by 35 percent of respondents as a top challenge among drug developers for phase I trials, second only to navigating regulatory compliance (- 38 percent).
Predictive biomarkers have a range of uses, including determining the likely benefits of a treatment and who is most likely to respond. This is often assessed by a companion diagnostic, which categorises patients as potential responders or non-responders based on a test or assay which detects predictive biomarkers, helping to enrich subsequent trial phases by filtering out patients less likely to benefit from the therapy.
Biomarkers based on the mechanism underlying a complex disease such as obesity, if validated as an endpoint, can potentially reveal more about a treatment’s efficacy.
Pharmacodynamic biomarkers indicate a biological response to treatment and can serve as surrogate endpoints if validated as consistently and accurately predictive of a particular clinical outcome. Biomarkers based on the mechanism underlying a complex disease such as obesity, if validated as an endpoint, can potentially reveal more about a treatment’s efficacy than broader endpoints such as body weight, while still providing key safety data. Such biomarkers can also encourage or dissuade continuation of treatment and make better use of smaller population sizes by accounting for the actual mechanistic impact of a treatment.
The context of use (COU) of biomarker assays must be firmly established, clearly and comprehensively citing how they should be used, and the regulated product development and review-related purpose of that use. A COU statement will typically include detailed information about how the biomarker will be measured, the analytical method involved and will be supported by various performance characteristics along with the specific clinical context wherein the biomarker is intended for use.
Pharmacology, dosing, endpoint selection and trial design
For use as endpoints, biomarkers must be correlated to a valid clinical outcome. That requires taking every possible step to characterise dosage and physiological responses. In early-stage trials, endpoints are typically selected based on short-term safety and dosing strategies informed by in vitro and in vivo models. However, standard preclinical safety assessment studies may not accurately predict adverse events in humans, particularly with emerging modalities like precision medicine targeting human antigens or genes not present in preclinical species. In these instances, biomarkers that are clearly and relevantly linked to therapeutic efficacy can potentially serve as clinical endpoints.
For use as endpoints, biomarkers must be correlated to a valid clinical outcome. That requires taking every possible step to characterise dosage and physiological responses.
One area with high potential for biomarkers is in pharmacology and therapeutic dose selection. Early trials have traditionally aimed to determine the maximum tolerable dose (MTD) to guide dosing in later phases. The emergence and adoption of clinically relevant biomarkers is gradually presenting alternatives to MTD, particularly around therapies such as cell and gene therapy (CGT) and immunotherapy. Novel mechanisms of action associated with CGTs naturally create dosing effects that apply in very different ways than with traditional small molecule pharmacotherapies. Rather than focusing solely on detecting adverse side effects, biomarkers can be monitored in patients to determine the optimal dose based on specific physiological responses, such as receptor occupancy.
As pharmacological indicators, biomarkers overcome the static nature of traditional in vitro cellular studies by providing more dynamic models of pharmacokinetic processes that reflect active biological mechanisms. For example, drug-drug interactions can be modelled more authentically using pharmacokinetic transporters as biomarkers of quantitative change in response to drug exposure.4 In addition, dynamic modelling of ‘safety’ biomarkers has the potential to provide predictive capabilities for more precise dosing, quantitative pharmacokinetic analysis, and to anticipate adverse reactivity.
Trial design and statistical methods are also key to determining the utility and validity of biomarkers. Different methods are applicable for determining whether patients are likely to benefit from a certain treatment and for assessing the success of an intervention.
To qualify as endpoints, biomarkers used in early phases must be relevant to later stages of drug development.
To qualify as endpoints, biomarkers used in early phases must be relevant to later stages of drug development. For emerging modalities and therapies with multi-indication targets, early studies can incorporate more precise, mechanism-driven endpoints, often using designs traditionally reserved for later-stage efficacy trials. Therapies targeting complex systems like the brain can benefit from early biomarker insights to help inform subsequent trial designs aimed at testing efficacy in multiple treatments and refining endpoints for later, pivotal stages. In this case, umbrella trials utilising master protocols and employing separate subtrials can benefit from biomarker-mediated characterisation of disease subtypes to help guide treatment assignment and in assessing drug efficacy. In early-phase studies, an umbrella trial could look for specific biomarker activity in addition to the broader safety and preliminary efficacy data, all of which informs which version of an investigational therapy is viable for subsequent phases.
More personalised or targeted therapeutics often involve smaller patient populations, particularly in early trials. As a result, adaptive trial designs and Bayesian methods are becoming increasingly valuable in optimising study outcomes. Rather than establishing a ‘null hypothesis’, Bayesian statistics use prior data to estimate the probability of future outcomes, allowing for more informed decision-making in clinical trials. Adaptive trials maximise insights from small study populations by allowing real-time adjustments to patient allocation, treatment arms and outcome predictions as new data emerges. Of course, the smaller the sample size, the more important it is not to over-interpret results or their implications.
Growing acceptance of biomarkers by regulatory bodies
Regulatory guidelines are just as crucial in early development as in later stages, though they allow for more flexibility in endpoints and investigational strategies. While biomarkers are increasingly being adopted as surrogate endpoints by the US Food and Drug Administration (FDA), advances in biomarker detection also present a variety of regulatory challenges related to the complexities in their selection, implementation and clinical interpretation.
Fortunately, regulators are showing interest in novel study designs and the introduction of new biomarkers as surrogate endpoints, opening the door to approval for as-yet underexplored therapies. Prior to being validated, the FDA will still consider biomarkers in the marketing approval process as a “reasonably likely surrogate endpoint” or “candidate surrogate endpoint.” Opportunities for engagement early in the drug development process, such as through the FDA’s INTERACT programme, can provide useful feedback prior to committing to a biomarker-driven development and clinical trial strategy for an investigational treatment. Such consultation is highly useful, particularly when preparing to launch trials with investigational therapies that, while promising, remain highly experimental.
Conclusion
As science advances, medical and regulatory frameworks continue to adjust and evolve to accommodate new tools and methods. As biomarkers become increasingly relevant in indicating the workings and effects of novel therapies, their potential as valuable clinical and regulatory endpoints is also gaining recognition. Biomarkers can play a crucial role throughout clinical development, especially in early phases. For novel therapies, they provide essential support in proving concepts, and advancing understanding of efficacy, safety and mechanism of action. All of this lays the groundwork for later phases, where regulators are becoming increasingly receptive to biomarkers as surrogate endpoints. The parallel evolution of novel therapies, biomarker endpoints and regulatory evolution may signal a new paradigm for clinical drug development over the coming years.
References
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US); 2016-. Co-published by National Institutes of Health (US), Bethesda (MD).
- Bagyinszky E, et al. (2020). Transcriptomics in Alzheimer’s Disease: Aspects and Challenges. International Journal of Molecular Sciences. 21(10):3517. https://doi.org/10.3390/ijms21103517.
- (2001) Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics. 69:89–95.
- Rodrigues AD. (2023). Reimagining the framework supporting the static analysis of transporter drug interaction risk; integrated use of biomarkers to generate pan-transporter inhibition signatures. Clinical Pharmacology and Therapeutics. 113:986–1002.
About the author


Clarke is a clinical pharmacologist with over 30 years’ experience in clinical research; seventeen of which are within industrial clinical pharmacology. Clarke trained in medicine and immunology at University College London and has had a long-standing interest in the utilisation of biomarker strategies in early clinical development. He has acted as Principal Investigator for over 350 early-phase studies including 17 FTIH monoclonal antibody studies. He is ex Chair of the ABPI Experimental Medicine Expert network and an Honorary Reader in Translational Medicine at the University of Manchester.
Related topics
Biomarkers, Clinical Trials, Drug Development, Drug Discovery Processes, Translational Science
Related conditions
Alzheimer’s disease, Cardiovascular disease, diabetes mellitus, HIV, Obesity
Related organisations
ICON Biotech
Related people
Dr Cyril Clarke (Vice President at ICON Biotech)