SBP scientists uncover mechanism for blood vessel regeneration
Posted: 23 November 2017 | Drug Target Review | No comments yet
A new study led by researchers at Sanford Burnham Prebys Medical Discovery Institute (SBP) has identified a signalling pathway that is essential for angiogenesis, the growth of new blood vessels from pre-existing vessels.
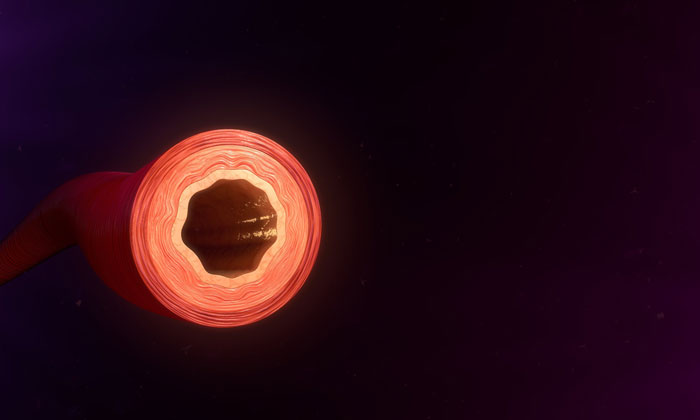

Their findings have the potential to improve current strategies to improve blood flow in ischemic tissue, such as that found in atherosclerosis and peripheral vascular disease associated with diabetes.
“Our research shows that the formation of fully functional blood vessels requires activation of protein kinase Akt by a protein called R-Ras, and this mechanism is necessary for the formation of the hallow structure, or lumen, of a blood vessel.” says Masanobu Komatsu, Ph.D., Associate Professor at SBP‘s Lake Nona campus.
“The findings are important because they shed new light on the biological process needed to increase blood flow in ischemic tissues.”
Possibility of improved blood flow in ischemia patients
Previous efforts to treat ischemia by creating new blood vessels have focused on delivering angiogenic growth factors such as vascular endothelial growth factor (VEGF) to ischemic sites. However, all of these studies, including more than 25 phase II and III clinical trials, have failed to offer significant benefit to patients.
Komatsu‘s research team used a combination of 3D cell culture and living tissue to show that VEGF promotes vascularisation, but the vessel structures formed are chaotic, unstable and non-functional. “Functional vessels need to have a lumen; a pipe-like opening that allows oxygenated blood and nutrients to travel through the body,” explains Komatsu, “and VEGF alone cannot fully support the formation of such a vessel structure.”
“Generating new blood vessels is similar to the way trees grow; sprouts develop from existing vessels and then branch out further and further to restore vascularity,” says Fangfei Li, Ph.D., Postdoctoral Associate in Komatsu’s lab and lead author of the paper.
“This study shows that there are distinct steps and signals that control the process.”
“First, VEGF activates Akt to induce endothelial cells to sprout. Then, R-Ras activates Akt to induce lumen formation,” explains Li. “The second step involving Akt activation by R-Ras stabilises the microtubule cytoskeleton in endothelial cells, creating a steady architecture that promotes lumen formation,” says Li.
“We propose that VEGF and R-Ras activation of Akt signalling are complementary to each other, both are necessary to generate fully functional blood vessels to repair ischemic tissue,” says Komatsu. “Our next step is to work toward promoting the combined signalling of Akt in clinical studies; prompting R-Ras activation through either gene therapy or pharmacologically in parallel with VEGF therapy,” concludes Komatsu.
Related topics
Cell Regeneration, Disease Research, Molecular Biology
Related conditions
Diabetes
Related organisations
Sanford Burnham Prebys Medical Discovery Institute (SBP)
Related people
Fangfei Li, Masanobu Komatsu