Succinate in cystic fibrosis lungs feeds deadly bacteria
Posted: 23 August 2019 | Rachael Harper (Drug Target Review) | No comments yet
Targeting a deadly bacteria’s use of succinate in the lungs could control infection and improve the health of people with cystic fibrosis.

A new study from Columbia University Vagelos College of Physicians and Surgeons has suggested the reason why the pathogen, Pseudomonas aeruginosa, thrives in cystic fibrosis lungs is due to the bacterium’s preference for succinate, a byproduct of cellular metabolism, that is abundant in cystic fibrosis lungs.
“Preventing infection by P. aeruginosa could greatly improve the health of people with cystic fibrosis,” said Sebastián A Riquelme, PhD, the study’s lead author and a postdoctoral fellow in the Department of Pediatrics.
And it’s possible that we could control infection by targeting the bacteria’s use of succinate in the lung.”
The excess succinate in the lungs of people with cystic fibrosis comes from an interaction between two proteins, CFTR and PTEN. Mutations in the CFTR gene causes cystic fibrosis by preventing the CFTR protein from transporting ions in and out of cells. But the mutations also disrupt CFTR’s interactions with PTEN.
The new study found that it is this abnormal PTEN-CFTR interaction that causes lung cells to release an excessive amount of succinate.
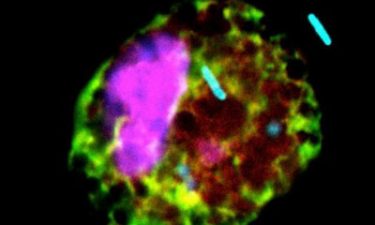

P. aeruginosa bacteria (blue) thrive in lungs that produce lots of succinate, a preferred food (credit: Sebastián Riquelme, Columbia University Irving Medical Center).
P. aeruginosa also actively adapts to the abundance of its favoured food. “Succinate-adapted bacteria divert their metabolism into the production of extracellular slime that makes the organisms extremely difficult to eradicate from the lung,” added the study’s senior author Alice Prince, MD, the John M. Driscoll Jr, MD and Yvonne Driscoll, MD Professor of Pediatrics.
The new findings suggest that it may be possible to treat P. aeruginosa infection by restoring the interaction between PTEN and CFTR, even if CFTR’s other functions are impaired.
New drugs for cystic fibrosis, such as the lumacaftor and ivacaftor combination currently available, restore the CFTR-PTEN interaction and may decrease the generation of succinate.
Limiting the accumulation of succinate may also reduce bacterial growth and adaptation. Succinate is mainly produced by immune cells during the inflammatory response, Riquelme says. “We predict that by controlling the exaggerated inflammation observed in the airway, we could reduce succinate and P. aeruginosa infection.”
The study was published in Science Translational Medicine.
Related topics
Analysis, Disease Research, Protein, Research & Development, Targets
Related conditions
Cystic fibrosis
Related organisations
Columbia University Vagelos College of Physicians and Surgeons, Science Translational Medicine
Related people
Alice Prince MD, Sebastián A Riquelme PhD