Developing a nanoparticle-delivered gene therapy for blinding eye disease
Posted: 8 July 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Ophthalmology and engineering combine in a novel nanoparticle-delivered gene therapy approach to treating wet age-related macular degeneration.

Researchers report they have successfully used nanoparticles to deliver gene therapies to the retina for blinding eye disease. According to the team, their uniquely engineered large molecules allow large genes to be compacted and delivered to the cells of the eye, with expression of the therapeutic gene evident several months after initial treatment.
Collaborative effort between an engineer and an ophthalmologist has demonstrated that nanoparticle-delivered gene therapies could be a possible treatment option for both wet age-related macular degeneration – an eye disease characterised by abnormal blood vessel growth that damages the retina in the back of the eye – and more rare, inherited retinal diseases that result in blindness.
The team said the nanoparticle approach was necessary because the viral vectors that most ocular gene therapies rely upon can create an unfavourable immune response that prevents repeated dosing. They also cannot carry the size of genes involved in the most prevalent inherited retinal degenerations, said Dr Peter Campochiaro, the Eccles Professor of Ophthalmology at the Johns Hopkins University School of Medicine and a member of the Johns Hopkins Medicine Wilmer Eye Institute.
The nanoparticle is formed when a biodegradable polymer surrounds and compacts long stretches of DNA. These nanoparticles are able to enter the cells of the eye, once inside the polymer degrade and the gene is released, so the cell can begin producing the therapeutic protein.
![In experiments in rats and mice, two Johns Hopkins scientists -- an engineer and an ophthalmologist -- report the successful use of nanoparticles to deliver gene therapy for blinding eye disease. A uniquely engineered large molecule allows researchers to compact large bundles of therapeutic DNA to be delivered into the cells of the eye [credit: John Hopkins Medicine].](https://www.drugtargetreview.com/wp-content/uploads/nanoparticle-gene-therapy-blindness-image-375x211.jpg)
![In experiments in rats and mice, two Johns Hopkins scientists -- an engineer and an ophthalmologist -- report the successful use of nanoparticles to deliver gene therapy for blinding eye disease. A uniquely engineered large molecule allows researchers to compact large bundles of therapeutic DNA to be delivered into the cells of the eye [credit: John Hopkins Medicine].](https://www.drugtargetreview.com/wp-content/uploads/nanoparticle-gene-therapy-blindness-image-375x211.jpg)
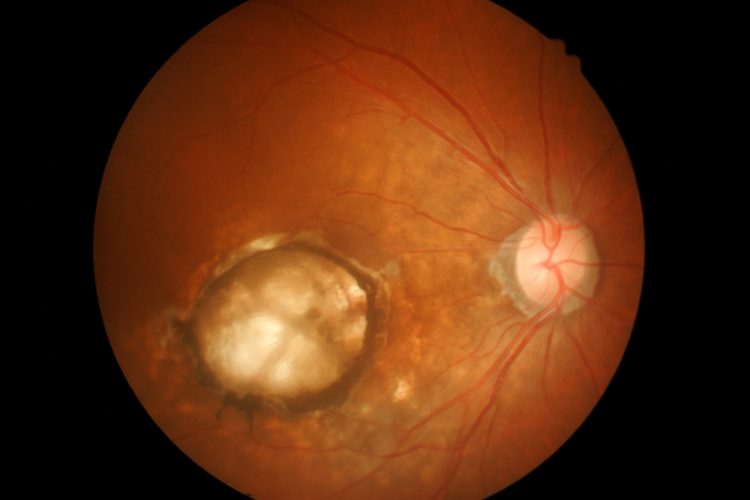
Retinal cells fluorescing due to the expression of the fluorescent protein. [credit: John Hopkins Medicine].
The ability of the nanoparticles to reach their target cell was confirmed when the researchers loaded them with a gene for a protein that causes cells to fluoresce and administered the treatment to rats. This experiment allowed the team to determine the location, amount and duration of gene expression achievable with the nanoparticles. According to the team, even eight months after treatment the majority of the retina cells in the rats’ eyes glowed, showing that the nanoparticles effectively deposited the florescent gene into the cells.
They then experimented with nanoparticles containing the gene for vascular endothelial growth factor (VEGF), which is responsible for the growth of abnormal blood vessels in people with wet macular degeneration. The researchers injected the eyes of 30 rats with these and determined the effects in the retina one, two and five months after injection.
After one month each rat had developed abnormal blood vessels under and within the retina, like those seen in patients with wet macular degeneration. This pathology was more extensive after two and five months, with emerging scarring under the retina that appeared similar to that seen in chronic untreated wet macular degeneration. Campochiaro explained: “These results show that the genes delivered by nanoparticles stayed active within the cells for several months.”
In a final test, the team evaluated the nanoparticle’s ability to deliver a therapeutic gene for wet aged macular degeneration. In this experiment the researchers injected nanoparticles loaded with a gene that produces a protein that neutralises VEGF into a genetically engineered murine model of the disease. Three weeks after injecting nanoparticles containing the gene for the anti-VEGF protein, the mice had a 60 percent reduction in abnormal blood vessels when compared to control mice. The same effect was seen 35 days later.
Dr Jordan Green, professor of biomedical engineering at the Johns Hopkins University School of Medicine, said: “These results are extremely promising. We have the ability to reach the cells most significantly affected by degenerative eye disease with non-viral treatments that can allow the eye to create its own sustained therapies.”
In clinic physicians currently inject similar proteins that block VEGF proteins into the eyes of people with macular degeneration, a treatment that helps control the overgrowth of abnormal, leaky blood vessels; however, the procedure has to be frequently repeated and so is burdensome for patients and caretakers alike.
An estimated 1.6 million people in the US with macular degeneration receive these ocular injections every four to six weeks. The team said a gene therapy treatment could allow prevent further vision deterioration with as little as a few initial treatments and that genetic diseases that cause blindness could be treated in a similar way, by introducing functional versions of genes that inherited mutations have disabled.
The paper was published in Science Advances.
Related topics
Drug Development, Gene Therapy, Genomics, Protein, Protein Expression, Proteomics, Regenerative Medicine, Research & Development, Therapeutics
Related conditions
age-related macular degeneration (AMD)
Related organisations
Johns Hopkins University School of Medicine.
Related people
Dr Jordan Green, Dr Peter Campochiaro