Targeting the immunotherapy potential of cytokines IL-12 and IL-18 with new advancements in protein engineering
Posted: 6 June 2023 | Dr Raymond Winquist (Alkermes) | No comments yet
In this article Dr Raymond Winquist, Oncology Fellow at Alkermes, covers the longstanding research challenges associated with cytokines: IL-12 and IL-18, and their untapped potential in immunotherapy and immuno-oncology.

Discoveries in recent years have firmly established immunotherapy as a major step forward in how we understand and treat cancer. We are in an era of immuno-oncology (IO) revolution with many approved therapies now available to treat a broad range of cancers. In this IO arena, we continue to search for additional treatments that can further benefit patients.
Generally, IO has been focused on harnessing the anti-tumour activity of certain cancer-fighting T-cells, a key cell type involved in the adaptive immune defense system. While several subsets of T-cells exist, most can be classified as either CD8+ or CD4+ T-cells. CD8+ T-cells, also called “cytotoxic T-cells,” are powerful, with an ability to kill cancer cells. Immune checkpoint inhibitors, a well-established class of IO treatments, are designed to improve the ability of T-cells to fight cancer by removing inhibitory or suppressive mechanisms that may dampen the anti-cancer functions of T cells. Natural killer (NK) cells are another immune cell type that, as the name suggests, also have potent cell-killing activity, and have a well-known role in the anti-tumour immune response.
Interestingly, both CD8+ T-cells and NK cells can be suppressed by another type of T-cell, known as a CD4+ regulatory T-cell (Tregs). In the context of a tumour microenvironment, Tregs are often present in high numbers, preventing an effective immune response to the tumour.
While immunotherapies such as immune checkpoint inhibitors have demonstrated practice-changing results in numerous types of cancer, there remain several types of cancers that do not have approved immunotherapy options.
Difficult-to-treat and rare, complicated cancers remain a perplexing challenge for IO researchers. For the last several years, the field has worked to unlock the potential of IO therapies for additional tumour types, and has explored options beyond checkpoint inhibitors.
One such approach has been through investigating the promise of certain immunomodulatory cytokines. Research has shown that these immune system proteins have the ability to trigger anti-cancer immune pathways. Studies also suggest that cytokines may augment the effectiveness of checkpoint inhibitors in certain settings. However, the full therapeutic potential of cytokines in treating cancers has not been fully explored.
Individual cytokines are differentiated in their functionality, requiring distinctive research design approaches best suited to their unique properties. Two powerful immunomodulatory cytokines in particular – interleukin (IL)-12 and IL-18 – are the subjects of current investigation. While the highly potent nature of their anti-tumour effects is widely established, clinical utility so far has been limited by severe challenges that persist despite extensive scientific inquiry across industry and academia.
At Alkermes, our interdisciplinary team of protein engineers, immunologists, pharmacologists, and analytical scientists is investigating the biology of several immunomodulatory cytokines including IL-12 and IL-18 to develop novel versions of these molecules with the goal of harnessing their therapeutic potential.
Exploring the anti-tumour potential of IL-12 as an IO therapy
Known for its ability to stimulate the activation of several types of immune cells, IL-12’s potent proinflammatory properties make it attractive as a potential cancer immunotherapy, both alone and in combination (with chemotherapy or other immunotherapy). However, the protein’s preclinical promise has not yet been replicated in clinical trials, where systemic administration is associated with dose-limiting toxicities and a narrow therapeutic index. Efficacy at tolerated doses so far has been disappointing.1
The biopharmaceutical industry is hard at work trying to overcome these challenges. Increasingly, IO researchers and biotech R&D teams are prioritising localised IL-12 delivery mechanisms that target tumours directly. Delivery of therapy is being evaluated through injections of DNA and RNA encoding IL-12, viral vectors, and other exploratory platforms designed to bring IL-12 in close proximity to the tumour and avoid excessive IL-12 in systemic circulation. Some approaches have already reached the clinical trial stage, with others not far behind.2
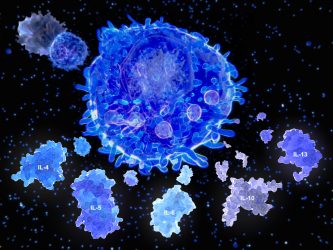

After activation by an antigen presenting cell, a T helper cell segregates the cytokines.
Through Alkermes’ innovative protein-engineering capabilities, we are investigating a potential approach to IL-12 administration that involves deconstructing a protein’s subunits and modifying them such that the individual subunits reach the tumour upon separate systemic injections. The tumour targeting of the subunits is achieved by adding a targeting moiety directed to a protein which is expressed in the tumour microenvironment in many solid tumours.
Once in the tumour, the subunits are designed to combine to form functional IL-12. The approach involves sequentially timed injections of each subunit, with the goal of minimising their systemic integration and hence prevent any substantial exposure to functional IL-12 outside the tumour. The sequential injection strategy is designed to add an additional level of safety, by increasing clearance of any unbound IL-12 subunits from systemic circulation after the first injection. We are now testing our lead molecules in advanced preclinical safety and efficacy studies to ascertain effective control of tumour growth without adverse tolerability issues.
IL-18: Anti-tumour potential and challenges
Like IL-12, IL-18 is a proinflammatory cytokine with stimulatory effects on several types of immune cells. In recent years, trials have shown IL-18 to be generally well-tolerated by patients as an immunotherapy option – also providing substantial evidence that higher doses of IL-18 can be generally tolerated by patients without the level of toxicities associated with IL-12 administration. Clinical development of IL-18 therapies has been curtailed, however, by the protein’s lack of efficacy. This lack of efficacy is attributed to a well-studied suppressive mechanism that prevents IL-18 from functioning. This suppressive mechanism involves a negative regulator – a protein called IL-18BP or IL-18 binding protein, that works as a “decoy receptor” by binding to IL-18 and preventing it from binding to its functional receptor.
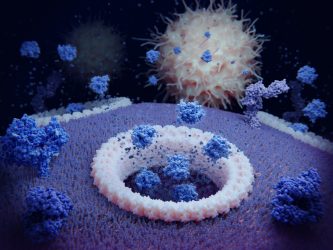

Pyroptosis: the cytokines IL1ß and IL18 are released through gasdermin D pores and attract immune cells. The foreground membrane proteins (dark blue) are T-cell receptors.
The clinical limitations stem from this endogenous negative regulator effectively shutting off IL-18’s known cancer-fighting immune response.3
Our team is working to engineer a novel variant of IL-18 designed to avoid binding with IL-18BP. Although the protein engineering approach is different, the rationale is related to the approach used to engineer our IL-2 candidate, nemvaleukin alfa, to maximise anti-tumor activity through prevention of binding to a negative regulatory receptor. Ultimately, our goal is for this approach to yield an enhanced cancer fighting response. We are currently selecting our lead IL-18 variant on the basis of potency for the IL-18 receptor, optimal pharmacokinetic profile and in vivo efficacy for inhibiting tumour growth.
Bringing therapies to patients who need them most
By using well-validated biology coupled with a differentiated approach to molecular design, we aim to increase therapeutic potential and allow for broad clinical utility of these cytokine approaches. Each cytokine is differentiated in its functionality and requires a distinctive approach suited for its unique property. We are focused on harnessing our expertise in molecular design and advanced protein engineering to continue designing novel biologic medicines that engage the immune system to fight cancer. We believe that the success of cytokines as a novel immunotherapy class relies on a combination of scientifically rigorous protein redesign, full understanding of the microenvironment challenges of different tumour types and clinical trials designed to demonstrate both monotherapy and combination potential.
Our goal is to design and develop potentially first- and best-in-class molecules to deliver meaningful clinical benefit across a wide range of cancers, including tumour types where immunotherapies are not yet approved or have not shown efficacy. Our deep interest in the interplay between immune cells, cytokines, and the tumour microenvironment has informed our clinical research to date as well as our IO pipeline program. By applying research and clinical insights from our investigational IL-2 development program, we aim to advance our IL-12 and IL-18 candidates through further preclinical studies and make important progress towards potential IO therapies.
Our vision is the same throughout the clinical development process – bring new options to patients who may need them the most.
Author Bio:


Ray is a drug discovery leader with over 30 years of drug discovery experience in both biotech and large pharma. Currently he is Alkermes’ Oncology Fellow and Head of a Oncology Preclinical Research at the company. He is also a member of the Translator Scientific Advisory Committee at Harvard Medical School, an Associate Editor at Journal of Pharmacology and Experimental Therapeutics, a member of the Editorial Board at Biochemical Pharmacology and Fellow of the American Heart Association (Council for High Blood Pressure Research).
References:
- M; LWR. Interleukin 12: Still a promising candidate for tumor immunotherapy? [Internet]. U.S. National Library of Medicine; [cited 2023 Jun 6]. Available from: https://pubmed.ncbi.nlm.nih.gov/24514955/
- Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA, et al. Localized interleukin-12 for cancer immunotherapy. Frontiers in Immunology. 2020;11. doi:10.3389/fimmu.2020.575597
- Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM, et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer [Internet]. American Association for Cancer Research; 2006 [cited 2023 Jun 6]. Available from: https://aacrjournals.org/clincancerres/article/12/14/4265/284841/Clinical-and-Biological-Effects-of-Recombinant
Related topics
Immuno-oncology, Immuno-oncology therapeutics, Immunotherapy, Oncology, Protein Expression, Proteomics
Related conditions
Cancer
Related organisations
Alkermes