New molecular insights on medical cannabis
Posted: 1 November 2023 | Andrew McCarthy (EMBL), Dr Jens Hausmann (EMBL), Dr Mathias Eymery (EMBL) | No comments yet
A study explores some key biological mechanisms that help explain the therapeutic effects of medicinal cannabis on inflammation and other disease states. Here, researchers from the European Molecular Biology Laboratory outline their investigations into the effect of tetrahydrocannabinol (THC) on the enzyme Autotaxin.


Autotaxin (ATX) is a 99-125 kDa lysophospholipase D involved in a large range of physiological and pathological processes.1 This critical enzyme is part of the nucleotide pyrophosphatase/ phosphodiesterase family and is also referred to as ENPP2. Five ATX isoforms have been identified, with ATX-b expressed in most human tissues and ATX-g specific to the central nervous system. ATX is mainly involved in phospholipidic metabolism and the production of extracellular lysophosphatidic acid (LPA) from lysophosphatidylcholine (LPC).2 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials.1-5
Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials.
Firstly, it has been shown that ATX is important for cancer progression and metastasis, as this enzyme is responsible for LPA generation.3,4,6 LPA is a growth factor that regulatesegulating many different cellular functions, some of which are important for malignant cells. Notably, it has been shown that LPA is a cell motility factor and inhibition of ATX results in a decrease of cellular invasion through a reduction of LPA concentration in the surrounding fluids in vitro.5 In vivo experiments and clinical trials showed that ovarian cancer cells produce high levels of LPA, as do other conditions such as pregnancy and acute coronary syndrome.3

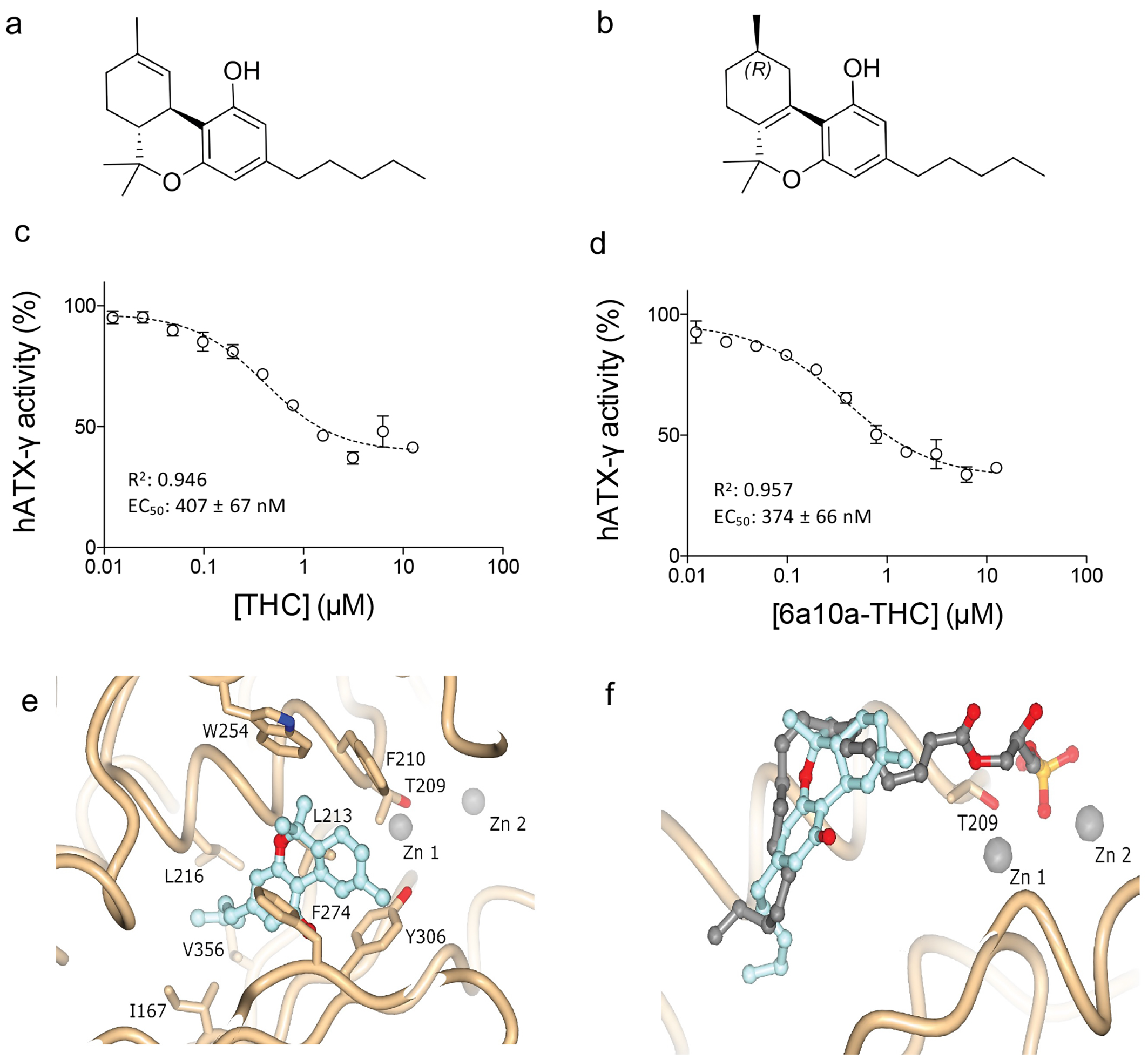
Recently, our laboratory discovered a potent partial inhibition of ATX by cannabinoids in a nanomolar range. The study opens new perspectives for ATX inhibitor development as well as a unique opportunity to understand the effects of medicinal cannabis on humans.11 The main psychoactive cannabinoid found in medicinal cannabis from the plant Cannabis sativa is Δ9-trans-tetrahydrocannabinol (THC) (Figure 1a), which can bind to cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2) at nanomolar concentration.12 The brain-specific ATX-g isoform and ATX-LPA signalling have been implicated in neuronal disorders such as multiple sclerosis, depression, Alzheimer’s disease, and neuropathic pain.13 Inhibition of ATX and reduced LPA production could explain some of the positive effects that medicinal cannabis exerts on neurological disorders, which are currently being assessed through clinical trials. We showed that for both ATX-b and ATX-g, THC works as a partial inhibitor with a magnitude of inhibition of 60 percent with LPC 18:1 (Figure 1a and b). The apparent EC50 values of THC with LPC 18:1 was 1026 ± 138nM for ATX-b and 407 ± 67nM for ATX-g (Figure 1c). To understand the inhibition of the studied cannabinoids towards ATX in more detail, we also performed biochemical analyses with the artificial THC derivative 9(R)-Δ6a,10a-tetrahydrocannabinol (9(R)-Δ6a,10a-THC). This material differs to THC in the position of the double bond in the C-ring (Figure 1a and b). Interestingly, this minimal difference impacts the apparent EC50 value, which is 844 ± 178nM for ATX-b and 374 ± 66 for ATX-g with LPC 18:1 as substrate (Figure 1d). The magnitude of inhibition is also higher, at approximately 75 percent (Figure 1c and d). This observation could suggest that 9(R)-Δ6a,10a-THC binding is stronger than THC and the best utilised cannabinoid inhibitor of ATX.

The results of this study open new perspectives for cannabinoid research and will hopefully provide a basis for including the monitoring of ATX and LPA levels during new clinical trials assessing medicinal cannabis use.
About the author


Mathias is currently doing his PhD at EMBL Grenoble, working on autotaxin inhibition by cannabinoids and development of new autotaxin inhibitors. He previously obtained a PharmD and a MSc degree from Grenoble Alpes University in 2019.


Andrew is a Team Leader at EMBL Grenoble since 2007. He has over 25 years of research experience in the application of structural biology techniques to understand protein function at the molecular level. His team mainly studies proteins involved in cell signalling and neuronal development.


Jens obtained his PhD from Leiden University for his work at the Netherlands Cancer Institute in Amsterdam, Netherlands. He is a researcher at University of Oldenburg, Germany. He formerly worked at EMBL as a postdoctoral fellow from 2017 to 2020 where he discovered the inhibition of ATX with THC.
References:
- Ninou I, Magkrioti C, Aidinis V. Autotaxin in Pathophysiology and Pulmonary Fibrosis. Frontiers in Medicine. 2018 Jun 13;5.
- Castagna D, Budd DC, Macdonald SJF, et al. Development of Autotaxin Inhibitors: An Overview of the Patent and Primary Literature: Miniperspective. J Med Chem. 2016 Jun 23;59(12):5604–21.
- Gotoh M, Fujiwara Y, Yue J, et al. Controlling cancer through the autotaxin–lysophosphatidic acid receptor axis. Biochemical Society Transactions. 2012 Feb 1;40(1):31–6.
- Lee D, Suh DS, Lee SC, et al. Role of autotaxin in cancer stem cells. Cancer Metastasis Rev. 2018 Sep;37(2–3):509–18.
- Umezu-Goto M, Kishi Y, Taira A, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. The Journal of Cell Biology. 2002 Jul 22;158(2):227–33.
- Valdés-Rives SA, González-Arenas A. Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development. Mediators of Inflammation. 2017;2017:1–15.
- Herr DR, Chew WS, Satish RL, Ong WY. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Molecular Neurobiology. 2020 Jan 1;57(1):372–92.
- Albers HMHG, Hendrickx LJD, van Tol RJP, et al. Structure-Based Design of Novel Boronic Acid-Based Inhibitors of Autotaxin. Journal of Medicinal Chemistry. 2011 Jul 14;54(13):4619–26.
- Keune WJ, Potjewyd F, Heidebrecht T, et al. Rational Design of Autotaxin Inhibitors by Structural Evolution of Endogenous Modulators. J Med Chem. 2017 Mar 9;60(5):2006–17.
- Miller LM, Keune WJ, Castagna D, et al. Structure–Activity Relationships of Small Molecule Autotaxin Inhibitors with a Discrete Binding Mode. J Med Chem. 2017 Jan 26;60(2):722–48.
- Eymery MC, McCarthy AA, Hausmann J. Linking medicinal cannabis to autotaxin–lysophosphatidic acid signaling. Life Sci Alliance. 2023 Feb;6(2):e202201595.
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). 2002 Feb;66(2–3):101–21.
- Keune WJ, Hausmann J, Bolier R, et al. Steroid binding to Autotaxin links bile salts and lysophosphatidic acid signalling. Nat Commun. 2016 Sep;7(1):11248.
Related topics
Molecular Biology
Related organisations
EMBL