Fibromyalgia pathogenesis provides drug target clues
Posted: 20 September 2016 | Kim Lawson (Sheffield Hallam University) | No comments yet
Fibromyalgia (FM) has been described as a condition of heightened generalised sensitisation to sensory input presenting as a complex of symptoms dominated by chronic widespread pain characterised by hyperalgesia and allodynia. A range of co-morbidities of variable intensity, such as fatigue, sleep disturbance, cognitive impairment, anxiety and depression, are often present…

The prevalence of this condition, which is more common in females than males, is reported to be 2-8% of the population and presents a major financial and social burden to patients and healthcare systems. Neuronal excitability associated with amplified responses of the central nervous system (CNS) to peripheral input leading to central sensitisation is believed to underlie the pathophysiology1,2. Peripheral nociceptive generators, such as nerve pathologies, neuro-inflammation, skeletal muscle abnormalities and ischaemia, play a role in the enhancement of the central components and the pain experienced by FM patients3,4.
People with chronic pain experience balance shifts to an enhanced excitation and reduced inhibition within the CNS. The enhanced excitability of the neurophysiology reflects altered neurotransmitter functionality and neuroplasticity augmenting sensory processing5 . Pharmacological treatments aimed at increasing antinociceptive neurotransmission in the CNS or lower levels of pronociceptive excitatory neurotransmitters are required. In the cerebrospinal fluid (CFS) of FM patients increased concentrations of substance P (two to three-fold), endogenous opioids (three to four-fold), glutamine (twofold), nerve growth factor (four-fold) and brain-derived neurotrophic factor (two to four-fold) have been observed1,6.
In contrast, the CFS levels of 5-hydroxy indoleacetic acid (5-HIAA) – the main metabolite of serotonin, and 3-methoxy-4-hydroxyphenethylene (MPHG) – the main metabolite of noradrenaline, as well as blood levels of L-tryptophan and serotonin are lower compared with healthy controls1,6. Thus, the changed biochemistry observed in FM patients is consistent with the proposed central neuronal excitability. The raised glutamatestimulating N-methyl-D-aspartic acid (NMDA) receptors in the dorsal horn of the spinal cord may be responsible for a wind-up phenomenon observed in FM patients.
Diffuse noxious inhibitory control

Fibromyalgia is dominated by chronic pain
In Fibromyalgia is dominated by chronic pain healthy subjects application of intense painful stimuli activates a whole-body analgaesia referred to as diffuse noxious inhibitory control (DNIC), which involves descending opioidergic and serotonergicnoradrenergic efferent pathways from the brain to the spinal cord that downregulate the pain signal. In FM, compared with healthy controls, DNIC is consistently reduced or absent7 . The altered biochemistry of serotonin and noradrenaline in the CSF and serum is consistent with a decreased endogenous serotonergic and noradrenergic activity responsible for the reduced DNIC in FM patients. In contrast, evidence from FM patients indicates normal or increased endogenous opioid activity with high baseline occupancy of the receptors, rather than a deficiency of endogenous opioid release6 . Thus, low-dose naltrexone (LDN), an opioid receptor antagonist, has been proposed as an effective treatment strategy in some FM patients8 . Microglia antagonism, rather than blocking endogenous opioid release, has been suggested as the mechanism by which LDN reduces the severity of the FM symptoms. Pro-inflammatory factors released from microglia, which in FM may be abnormally sensitised, interact with CNS neurons leading to central facilitation of pain processing9.
Pharmacological treatments that simultaneously raise both serotonin and noradrenaline, for example, tricyclic antidepressants and serotonin noradrenaline reuptake inhibitors have gained interest and demonstrated efficacy in treating FM, further supporting the involvement of the low levels of these neurotransmitters in the pathogenesis of the disease2 . Treatment with the tricyclic antidepressant amitriptyline also correlated the clinical response with normalisation of function within the bilateral thalamus and basal ganglia, demonstrating involvement of serotonin and noradrenaline in several central aspects of the pathophysiology of FM. In contrast, drug treatments (fluoxetine, paroxetine, trazodone, esreboxetine, droxidopa) that selectively raise either of the neurotransmitter levels, e.g., selective serotonin reuptake inhibitors and noradrenaline reuptake inhibitors, have not demonstrated the success of the broader spectrum interventions2 . Interestingly, when amitriptyline was administered in combination with melatonin a superior improvement in symptoms was observed compared with amitriptyline alone10. Thus, stimulation of melatoninergic receptors improved the serotonergic-noradrenergic components of the descending endogenous pain-modulating system and, thereby, abnormality of the melatoninergic system may also play a role in the pathogenesis of FM.
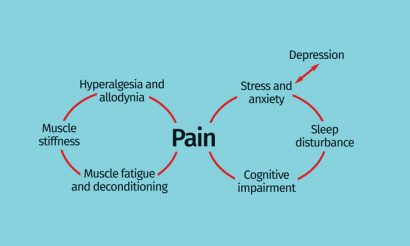

Figure 1: Interrelationship of centrally- and peripherally-derived symptoms of fibromyalgia
NMDA receptor
The enhanced wind-up and central sensitisation in FM has been associated with elevated excitatory CNS neurotransmitters. Glutamate levels in FM patients are elevated in key pain-processing areas of the brain and these levels change in response to successful treatment that attenuates the pain11,12. For example, glutamatergic activity in the insula was decreased by pregabalin, a voltage-gated calcium channel α2δ subunit ligand, with those FM patients with the highest levels of glutamate before treatment being the most likely to respond to the drug13. Calcium ions enable neurotransmitters to bind to vesicles at the presynaptic membrane terminal, thus inhibiting the α2δ subunit, and results in decreased calcium influx and a reduction in the release of neurotransmitters that transmit nociceptive signals between neurons.


The enhanced wind-up and central sensitisation in FM has been associated with raised excitatory neurotransmitters
In addition, a subset of FM patients responded to NMDA receptor antagonists (ketamine, dextromethorphan and memantine) which would be consistent with suppression of an increased glutamatergic activity, however such treatments are often not well tolerated and have limited use in the clinic2,6. Improved understanding of the contribution of the activity of NMDA receptor subtypes in pain – and the other symptoms of FM – and recent development of subtypespecific modulators should address those limitations of this therapeutic approach14. In addition to reducing pain, the α2δ subunit ligands pregabalin and gabapentin have been reported to improve other symptoms characteristic of FM, such as sleep, anxiety and fatigue, supporting the involve ment of the neurotransmitter imbalances (e.g., glutamate) targeted by these drugs in the co-morbid symptoms15.
Identification of subtypes of the α2δ subunits, α2δ-1 and α2δ-2, have shown that analgaesic effects are primarily due to ligands binding to the former, whereas binding to the latter subtype appears to contribute to CNS side effects16. Evaluation of the preferentially α2δ-1 subunit selective ligand mirogabalin (DS5565) demonstrated a preferable efficacy profile in a treatment study of diabetic peripheral neuropathic pain17. These findings support testing of mirogabalin in clinical trials with FM patients, where clinically meaningful differences in efficacy and safety may provide a more successful therapeutic option.
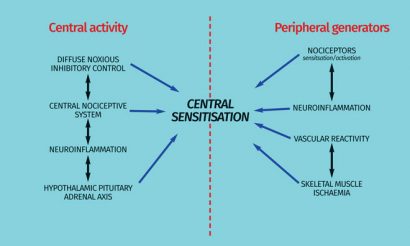

Figure 2: Central and peripheral mechanisms related to the pathophysiology of fibromyalgia
Although efficacy is observed with modulation of bioamines or the α2δ ligands in the management of FM symptoms, the outcomes are often limited with a partial reduction of symptoms only in a proportion of patients. Thus, the potential of greater benefit or more preferable outcomes from combined therapies targeting multiple pharmacological mechanisms has been considered. In a single-blind randomised trial, the combined use of pregabalin plus paroxetine, amitriptyline or venlafaxine resulted in significantly lower somatic symptom and depression scale scores; better life satisfaction; mood and sleep quality; higher medication tolerability and less frequent adverse effects in patients with FM18. Suppression of neuronal excitability and neurotransmitter release appears to be a key pharmacological property to the management of FM as observed with the α2δ ligands and the bioamine modulators. Thus, anticonvulsant drugs such as levetriactem, topiramate and lacosamide, which suppress neuronal hyperexcitability by a mechanism other than α2δ subunits have been evaluated as a potential treatment approach, but have failed to provide sufficient evidence of efficacy against the symptoms of FM19. The mechanism(s) of action of the gabapentanoids, pregabalin and gabapentin appear to provide a unique, albeit limited, control of the neuronal hyperexcitability and central sensitisation associated with FM.


Cannabinoids such as nabilone and dronabinol improved quality of life in FM patients in clinical trials
Although raised substance P levels have been reported in FM patients, substance P antagonists have provided inconsistent outcomes or failed in clinical trials in FM and other chronic pain states2,6, casting questions about how critical this neurotransmitter is as a drug target in pain transmission. Interestingly, topically applied capsaicin significantly improved the symptoms of FM20. Capsaicin binds to the transient receptor potential vanilloid 1 subunits (TRPV1) located in peripheral nociceptors and desensitises nociceptive processes, possibly due to depletion of substance P21. These findings infer, at least, a peripheral role of substance P in the regulation of peripheral generators and sensory factors activating central neuronal mechanisms responsible for the symptoms of FM.
Dopamine deficiency and endocannabinoid deficiency in the CNS, where there is an involvement in regulation of pain processing and chronic stress, have also been implicated in the pathophysiology of FM22,23. In patients with FM dopamine release into the basal ganglia in response to painful stimuli is attenuated or absent22. Attempts to rectify this dysfunction with dopamine receptor agonists have been inconsistent with pramipexole improving symptoms but similar outcomes have not been observed with ropinirole and terguride24-26. Evaluation of cannabinoids, such as nabilone and dronabinol, as a treatment approach demonstrated in clinical trials an improvement of quality of life in patients with FM, but with limiting adverse effects2 . Psychotropic effects due to hepatic metabolites from first-pass metabolism could limit the clinical utility of cannabinoids, which has led to the evaluation of a trans – dermal application of the D-(-)-glyceric acid ester of delta-9-tetrahydrocannabinol, ZYN001 in FM patients27.
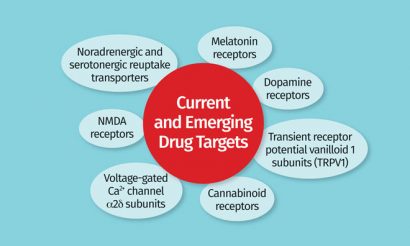

Figure 3: Current and emerging drug targets for treatments of fibromyalgia
Systemic stress-related effects associated with alterations in the hypothalamic pituitary adrenal axis, and autonomic and cardiovascular system have also been suggested to enhance or underlie the symptoms, particularly the pain, of FM2,6. Although studies in FM generally have shown alterations of these stress systems, the findings of the studies are often inconsistent with abnormal HPA or autonomic function in only a small percentage of patients, with significant overlap between patients and healthy subjects. Further data also suggests that HPA and autonomic abnormalities occur as a consequence of the pain in FM patients28.
Although inflammation has also been suggested to underlie the pathology in FM, literature on cytokines has been variable and the studies have several limitations that could influence the findings1,2,6. Pro-inflammatory cytokines might play a role in the generation and enhancement of the chronic pain characteristic of FM, however evidence does not as yet allow conclusions to be drawn as to whether the inflammatory response is the cause of the symptoms or due to the changed physiology initiating the pain. It is important to note that anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs and steroids have been shown in clinical trials to have no or very limited efficacy in the treatment of FM2,6.
Conclusion
Advances in the understanding of the pathophysiology of FM are providing clues as to underlying mechanisms as targets for new medications (Figure 2). The alteration of multiple systems and biological mechanisms, both at central and peripheral levels, has often introduced confusion as to which processes are a cause or consequence of FM. Bioamine modulation and voltage-gated Ca2+ channel α2δ subunits in addition to dopamine receptors, NMDA receptors, cannabinoid receptors and melatonin receptors are emerging as drug targets (Figure 3). However, the inconsistency of clinical data for drugs that target single mechanisms support the requirement of therapies that modulate more than one dysfunctional neurotransmitter.
The diversity of pharmacological targets gaining interest emphasises the complexity of FM, but insight into potential drug treatment profiles offers important clues for condition-focused therapies and improved diagnosis. Further evidence of the symptoms indicates heterogeneity with multiple potential aetiologies. Such an interpretation is consistent with an heterogeneity of drug treatment outcomes often leading to the requirement of individualised management. Finally, greater understanding of the central and peripheral contributions to the pathophysiology is required so that therapies can be targeted toward both components to manage the characteristic symptoms, but also to consider accompanying co-morbid conditions.


Related topics
Cannabinoids, Drug Targets
Related conditions
Fibromyalgia
Related organisations
Sheffield University
Related people
Kim Lawson