SRP-001: redefining pain treatment with a safer, non-opioid analgesic
Posted: 1 July 2024 | Dr Hernan A Bazan (South Rampart Pharma) | No comments yet
This Q&A with Dr Hernan A Bazan, CEO and Co-founder of South Rampart Pharma, Inc, discusses the potential merits of novel non-opioid analgesic SPR-001, which is Phase II-ready for acute and neuropathic pain and migraines. Its benefits mean it is poised to disrupt the $87 billion pain management market and address significant clinical and societal unmet needs.

Can you explain the mechanism of action of SRP-001 and how it differs from traditional analgesics like acetaminophen (ApAP) and non-steroidal anti-inflammatory drugs (NSAIDs) in treating acute, chronic and neuropathic pain?
SRP-001 targets the central nervous system (CNS) by producing N-arachidonoylaminophenol (AM404) in the midbrain’s periaqueductal grey (PAG) region, crucial for pain sensation and regulation. Unlike acetaminophen (ApAP), which metabolises into the hepatotoxic compound N-acetyl-p-benzoquinone imine (NAPQI), SRP-001 does not generate harmful byproducts, reducing liver damage risk. Additionally, unlike NSAIDs that inhibit cyclooxygenase enzymes (COX-1 and COX-2) and can cause gastrointestinal (GI) and renal side effects, SRP-001 avoids these pathways, alleviating the risk of GI bleeding and kidney injury. As a non-opioid, SRP-001 also eliminates abuse potential, positioning it as a safer and effective drug candidate for the treatment of acute and neuropathic pain and migraine headache.
How does SRP-001’s safety profile, especially concerning hepatotoxicity and nephrotoxicity, compare to other common pain medications?
SRP-001 could provide a distinct safety advantage over common analgesics. Our studies showed that SRP-001 does not produce the hepatotoxic metabolite NAPQI, a leading cause of acute fulminant liver failure in the US, UK, and many other parts of the Western world. SRP-001 has a benzenesulfonamide linker that bypasses high doses of NAPQI formation and liver injury. Moreover, SRP-001 preserves hepatic tight-junction integrity even at high doses, a second mechanism for avoiding liver injury. As a non-NSAID, it reduces adverse effects associated with these pain relief drugs, including kidney injury, GI gastritis/ulceration and blood pressure elevation, which can affect patients with cardiovascular disease and the elderly. We set out to improve health by developing a novel non-opioid that avoids potential abuse. Since we know how it works in the CNS for pain relief, and SRP-001 will have a higher therapeutic index due to this robust safety profile, we aim to be able to treat moderate pain effectively.
Can you discuss the single-cell transcriptomic analysis presented in the paper and its implications for understanding the pain-relief mechanisms of SRP-001?
The single-cell transcriptomic analysis in the Sci Reports paper1 provides insights into SRP-001’s pain-relief mechanisms. Using the 10X Genomics Chromium platform, we conducted ribonucleic acid (RNA) sequencing on the midbrain’s PAG region in animals treated with SRP-001, ApAP and a vehicle control, focusing on gene expression changes related to pain processing.
We found that SRP-001 modulates genes in the endocannabinoid signalling pathway, including fatty acid amide hydrolase (FAAH), 2-Arachidonoylglycerol (2-AG), cannabinoid receptor 1 (CNR1), cannabinoid receptor 2 (CNR2) and transient receptor potential cation channel subfamily V member 4, TRPV4. SRP-001 downregulates FAAH, increasing endocannabinoid levels and reducing pain perception. It also elevates AM404 levels, enhancing TRPV1 and cannabinoid receptor effects. Additionally, computational modelling via CellChat and pathway enrichment analysis revealed enhanced pain modulation pathways in SRP-001-treated samples, distinguishing it from ApAP. Overall, SRP-001’s modulation of pain signalling genes and pathways through endocannabinoid enhancement and FAAH inhibition supports its potential as an effective non-opioid pain therapeutic, validating the planned clinical trials.
Could you elaborate on the potential market size for SRP-001 and how it fits into the broader landscape of pain therapies?
The global pain management market is projected to reach about $87 billion by 2025, driven by rising chronic diseases, an ageing population and more surgical procedures. Despite its size, there is a significant unmet need for effective, non-addictive pain relief due to the opioid crisis and NSAIDs’ side effects, such as GI, kidney and cardiovascular risks.
SRP-001 is a novel non-opioid pain relief candidate that works centrally in the brain, offering robust pharmacokinetics without the adverse effects of current medications. It addresses both acute and chronic pain, avoiding the addiction risks associated with opioids. Initially targeting acute pain, SRP-001 is also being evaluated for chronic conditions like neuropathic pain and migraines, potentially broadening its market reach.
Effective commercialisation strategies will be essential, including pricing, formulary placements, and legislative support. Engaging healthcare providers, payers and patients to highlight SRP-001’s non-addictive nature and safety benefits is crucial. Demonstrating real-world effectiveness will be vital to gaining stakeholders’ trust.
By addressing critical gaps in current pain management with an effective and safe non-opioid, SRP-001 could significantly disrupt the market and capture a profitable portion. With a strong market entry strategy and continuous evidence generation, SRP-001 has the potential to transform pain therapy and meet the enormous unmet needs in this field.
How does SRP-001 address the current limitations and challenges associated with opioid and non-opioid pain therapies?
SRP-001 offers a novel, non-opioid solution. Opioids carry high addiction risks and severe CNS side effects like sedation and respiratory depression. Non-opioids like acetaminophen and NSAIDs have issues including hepatotoxicity, nephrotoxicity and gastrointestinal damage. SRP-001 avoids these problems due to its non-opioid nature, preventing addiction and severe CNS side effects. Thanks to its non-hepatotoxic and non-NSAID profile, it mitigates the risk of liver, kidney and GI complications.
In Phase I clinical trials, SRP-001 showed no serious adverse events among 56 healthy volunteers, highlighting its safety. It offers rapid uptake and sustained effects, ensuring prolonged analgesic relief and reducing the need for frequent dosing, thus improving patient compliance. By triggering AM404 formation in the midbrain’s PAG region, SRP-001 provides a novel pain relief mechanism, regulating crucial pain-related genes and signalling pathways.
SRP-001’s innovative approach positions it as a potential game changer in pain management, addressing unmet needs and offering a safer, more effective alternative to existing therapies. As it progresses to Phase II trials, SRP-001 is set to significantly impact the pain management market, drawing interest from investors, healthcare providers and patients.
Figures
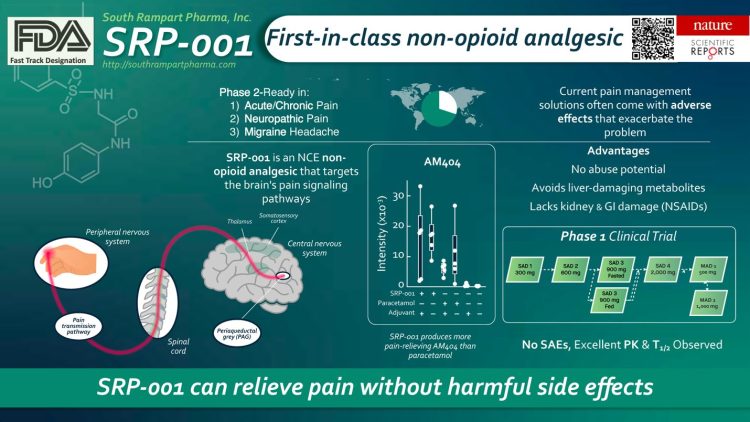

Figure by South Rampart Pharma, illustrating the properties of SRP-001.
Reference
1 Bazan H A, et al. Transcriptomic signature, bioactivity and safety of a non-hepatotoxic analgesic generating AM404 in the midbrain PAG region. Scientific Reports 14, 11103 (2024). https://doi.org/10.1038/s41598-024-61791-z
About the author


Dr Bazan is the CEO and Co-founder of South Rampart Pharma, Inc. He is spearheading the scientific and clinical development and fundraising of South Rampart Pharma and technology development efforts. As an academic vascular surgeon, he is the John Ochsner Endowed Professor of Surgery in Innovation. His clinical interests include treating carotid artery disease and acute stroke, using minimally invasive approaches to aortic aneurysms and peripheral arterial disease, and managing acute post-operative pain. South Rampart Pharma, Inc. was founded in 2016 to develop safer, non-opioid therapies for acute and chronic pain. Dr Hernan Bazan authored the first manuscript describing the library of analgesic compounds’ synthesis, lack of hepatoxicity, analgesic characterisation, and antipyretic properties, published in September 2020 in the European Journal of Medicinal Chemistry and a current paper under advanced review (open access) in Nature Communications.
He is the PI or Co-I on two NIH/NINDS STTR (small business commercialisation) grants to develop a novel non-narcotic and non-toxic analgesic to safely treat acute, chronic and neuropathic pain. He is the co-inventor of seven issued or pending patents and has authored over 50 publications. He and his team have completed Phase I clinical trials, paving the way for Phase II RCTs in acute and neuropathic pain. He was bestowed the 2024 NIH HEAL Director’s Trailblazer Award for this work.
Dr Hernan Bazan received a BS in molecular biology from Vanderbilt University and has spent two years in medical school as a Howard Hughes Medical Institute (HHMI) Research Scholar at the NIH. He earned an MD from Georgetown University, followed by a General Surgery Residency at Mt. Sinai Hospital, New York, and a Vascular Surgery Fellowship from Yale University.
Related topics
Analysis, Central Nervous System (CNS), Clinical Trials, Targets, Therapeutics, Toxicology
Related conditions
Migraine, Neuropathic pain
Related organisations
South Rampart Pharma Inc.
Related people
Dr Hernan A Bazan (South Rampart Pharma)