The essential role of recombinant phage display antibody libraries
Posted: 5 November 2024 | John Cardone (Bio-Rad Laboratories) | No comments yet
The emergence of new antibody-based therapeutics, such as antibody-drug conjugates, alongside a continued drive towards alternatives to animal testing approaches, highlights the need for precise and sensitive tools enabling the identification and characterisation of the best drug candidates for clinical progression. Capable of keeping up with the evolving drug development field, recombinant antibody phage display libraries provide flexibility to support new technologies and complex drug modalities in preclinical research.

Since the 1970s, when hybridoma technology enabling the generation of monoclonal antibodies (mAbs) was first developed,1 antibody-based therapeutics have become one of the most rapidly growing drug categories, with applications across cancer indications, immune disorders and infections. In 2024 alone, almost 50 antibody drug candidates are anticipated to enter regulatory review, the majority of which are mAbs.2 The industry has also started to embrace novel innovative antibody-based drug modalities, with the inclusion of numerous bispecific antibodies and antibody-drug conjugates (ADCs), for lung and breast cancer respectively, as well as a mixture of two immunocytokines for skin cancer.
Aside from the advent of complex antibody-based drugs, the industry is facing some additional changes which are shaping drug development. Recently, the FDA announced that new medicines need not be tested in animals to receive regulatory approval.3 This change signals a major shift away from animal use in drug safety regulation and encourages the use of alternative methods like organ-on-chip or other animal-free technologies.
In light of these key developments, the need for flexible yet highly specific and sensitive tools that provide vital information on a drug’s safety and efficacy are essential to help determine the best drug candidates for preclinical and clinical progression.
Overcoming traditional antibody generation limitations with recombinant technologies
Antibodies remain essential tools in this ever-changing field for preclinical bioanalysis and drug monitoring through ligand-binding assays (LBAs). These assays may include pharmacokinetic (PK) assays, which provide information on the drug’s properties, and immunogenicity assays for the detection of anti-drug antibodies (ADA), which can lead to adverse events and reduced efficacy. When the drug is a human or humanised monoclonal antibody, developing drug monitoring assays presents specific challenges due to the abundance of human antibodies in patient serum. Therefore, PK assays require highly specific detection reagents that can bind to the drug without interacting with similar immunoglobulin molecules. Additionally, a well-designed ADA assay needs a highly specific reference antibody to measure the anti-drug response accurately.
While polyclonal antibodies are cost-effective, their batch-to-batch variability leads to inconsistent results.4 mAbs offer better consistency, but a study found that nearly a third of hybridoma cell lines contained extra heavy or light chains, compromising their reliability.5 Additionally, genetic drift in hybridoma cell lines can lead to loss of antibody binding or expression over time. Anti-idiotypic antibodies (which specifically target the idiotope of another antibody) are considered optimal for PK assays and as surrogate positive controls or reference standards in ADA assays.6 However, traditional sources like rodent monoclonal anti-idiotypic antibodies and primate polyclonal serum pose several challenges. Identifying rare specificities for drug-target complex-specific antibodies and determining the affinities of immunoglobulin G (IgG) antibodies generated through immunisation is particularly difficult. Moreover, ethical considerations regarding the use of laboratory animals further complicate the use of primate polyclonal serum, and successful immunisation is not always guaranteed.
The creation of sophisticated human antibody libraries covering a vast immune repertoire through de novo synthesis, such as HuCAL (human combinatorial antibody library),7 combined with phage display technology and optimised for in vitro expression, have revolutionised the development of recombinant monoclonal anti-idiotypic antibodies.8 These libraries enable the generation of antibodies enhancing biotherapeutic analysis during preclinical and clinical development and routine drug monitoring, while also addressing common antibody production challenges. Generated using entirely in vitro processes, recombinant anti-idiotypic antibodies offer greater predictability, flexibility and optimisation potential, ensuring a long-term supply of sequence-defined, fully human antibodies without the use of animal-derived components or laboratory animals.8 In vitro selection methods also enable the isolation of antibodies targeting specific epitopes, including phosphorylated residues or point mutations, for precise detection or inhibition of target molecules, and address ethical concerns relating to animal immunisation with toxins in ADC development.
Innovations in anti-idiotypic antibody development and diverse applications
Phage display libraries and guided selection strategies have successfully generated anti-idiotypic antibodies with varying affinities and different binding modes specific to different forms of antibody drugs (eg, free, target-bound) (Figure 1).8 Selection in the presence of isotype subclass-matched antibodies produces paratope-specific (ie, antigen-binding site-specific) anti-idiotypic antibodies, termed Type 1, which inhibit drug-target binding and are useful for measuring free drug concentrations and as positive controls in ADA assays. Alternatively, non-inhibitory anti-idiotypic antibodies, termed Type 2, are selected for idiotope specificity outside the antigen-binding site, detecting total drug-free, partially bound and fully bound. Through refined guided selection strategies, drug-target complex-specific antibodies, termed Type 3, can be selected by using the drug-target-bound antibody as bait and blocking the library with individual complex components. Finally, Type 4 antibodies bind to a complex between a drug antibody and a Type 1 antibody.
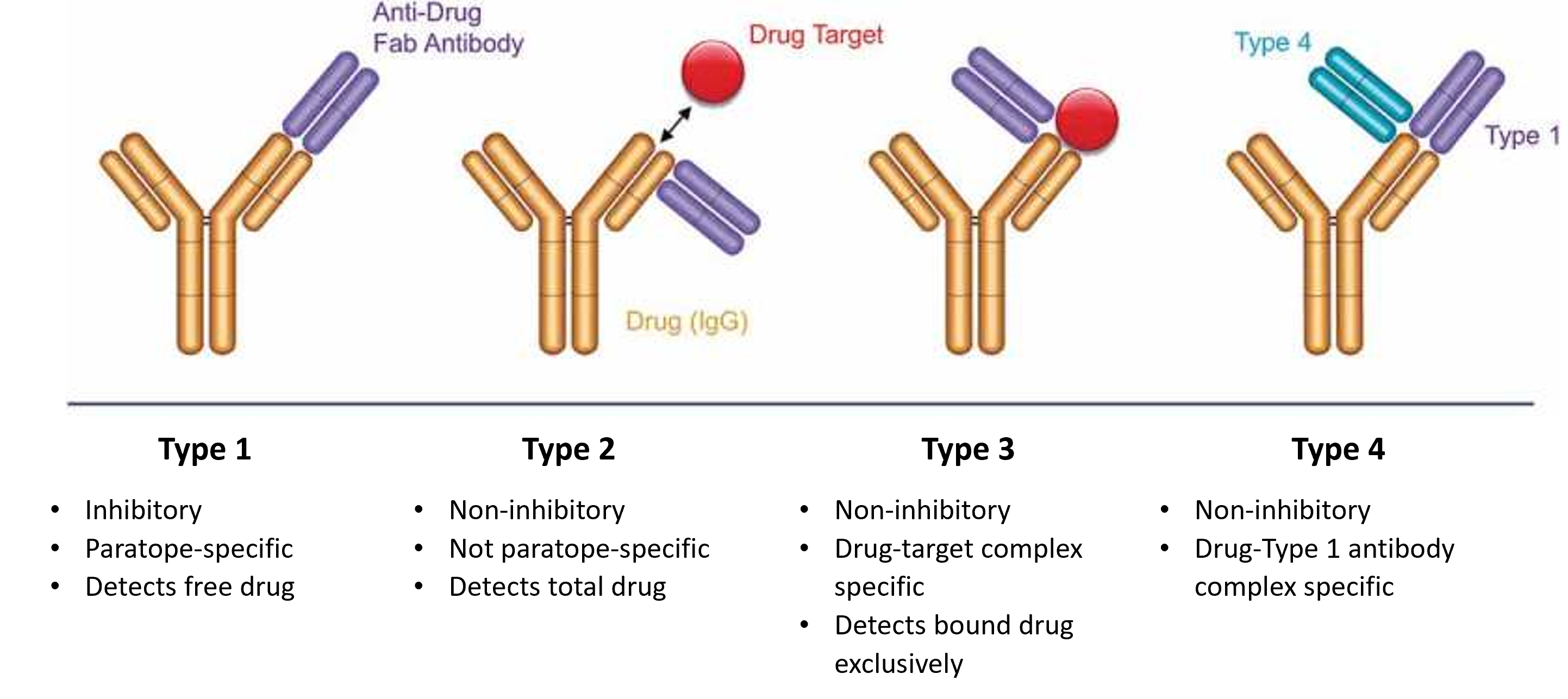

Figure 1. Alternative binding modes of anti-idiotypic antibodies generated using an antibody phage display library in combination with guided in vitro selection. Adapted from Harth S et al. (8) under a CC BY-NC-ND 4.0.
This comprehensive toolbox of Type 1, Type 2, Type 3 and Type 4 anti-idiotypic antibodies provides PK and ADA assay developers with enhanced flexibility for a thorough analysis of antibody candidates. For example, Type 3 antibodies with antigen-capture ELISA enable detection of monovalent Fab fragments, such as ranibizumab, which is otherwise undetectable using Type 1 antibodies in an ELISA bridging format due to its single binding domain.8
Keeping up with evolving strategies in preclinical bioanalysis
Recombinant anti-idiotypic antibodies, generated in vitro through sophisticated antibody libraries in combination with phage display technology, provide a reproducible, long-term supply of highly sensitive and specific detection reagents and controls needed for preclinical assays. These antibodies ensure predictability, flexibility and optimisation potential, addressing common production challenges, while eliminating the need for animal-derived components.
References
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug;256(5517):495–7.
- Crescioli S, Kaplon H, Chenoweth A, et al. Antibodies to watch in 2024. mAbs. 2024 Jan 5;16(1).
- Wadman M. FDA no longer needs to require animal tests before human drug trials [Internet]. www.science.org. 2023. Available from: https://www.science.org/content/article/fda-no-longer-needs-require-animal-tests-human-drug-trials
- Baker M. Reproducibility crisis: Blame it on the antibodies. Nature. 2015 May;521(7552):274–6.
- Bradbury ARM, Trinklein ND, Thie H, et al. When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. mAbs. 2018;10(4):539–46.
- Harth S, Frisch C. Recombinant Anti-idiotypic Antibodies in Ligand Binding Assays for Antibody Drug Development. Methods in Molecular Biology. 2021;291–306.
- Prassler J, Thiel S, Pracht C, et al. HuCAL PLATINUM, a Synthetic Fab Library Optimized for Sequence Diversity and Superior Performance in Mammalian Expression Systems. Journal of Molecular Biology. 2011 Oct 1;413(1):261–78.
- Harth S, Ten Haaf A, Loew C, et al. Generation by phage display and characterization of drug-target complex-specific antibodies for pharmacokinetic analysis of biotherapeutics. mAbs. 2018 Dec 5;11(1):178–90.
About the author
John Cardone, PhD, Marketing Manager – Custom Antibodies at Bio-Rad Laboratories
John Cardone is the Marketing Manager for Bio-Rad’s Custom Antibody Services. He manages the Pioneer™ Antibody Discovery Platform for biotherapeutic lead generation and the HuCAL® service for bioanalytical antibodies. Prior to this, he worked at an in vitro immuno-diagnostic company as Strategic Marketing Manager. John holds a PhD in immunology from Kings College London and an MBA from Warwick Business School, both in the UK.
Related topics
Antibodies, Assays, Drug Development, In Vitro
Related organisations
Bio-Rad Laboratories
Related people
John Cardone (Bio-Rad Laboratories)