Rapid delivery of toxicological material
Posted: 10 September 2024 | Alejandro Fernandez Martell (Lonza), James Berrie (Lonza) | No comments yet
With increasingly rapid timelines for achieving IND-ready processes, a bottleneck has emerged in the provision of toxicology data to support INDs for new therapeutics and hence delivery of FIH studies, explains Alejandro Fernandez Martell and James Berrie from Lonza.


The current landscape of protein drug development is characterised by accelerated timelines where new drugs are approved in months rather than years. To achieve an aggressive timeline from DNA to investigational new drug (IND) application, drug developers have strengthened collaborations with contract development & manufacturing organisations (CDMOs) to expedite drug development. In recent years, the biopharmaceutical industry has identified rapid Toxicology (Tox) material supply as the new critical path to IND readiness.
Rapid delivery of IND-enabling Tox testing is crucial for a timely IND submission
Advanced technologies enabling increasingly rapid timelines from vector construction to good manufacturing practice (GMP) manufacture brings the prospect of ever shorter timelines to IND readiness. However, developing an accelerated IND application requires that good laboratory practice (GLP) toxicological data must be generated, collected, interpreted and integrated in the IND-enabling data package. Hence, in many cases an earlier IND may be prevented by the timely provision of representative Drug Substance (DS) to execute such toxicology studies.
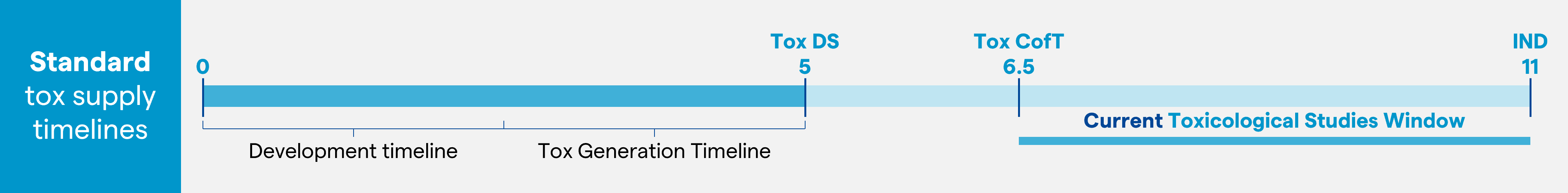
Drug developer companies across the pharma industry report notable reductions in drug development timelines where 10 to 12 months, from lead monoclonal antibody (mAb) identification to IND application, is the new norm and in which earlier Tox material generation has been a critical component for shortening IND timelines. In our experience, reaching a rapid drug substance material delivery to support toxicology studies is becoming a major milestone on the Phase I CMC clinical development path (Figure 1).
Reduce preclinical failures with smarter off-target profiling
24 September 2025 | 15:00PM BST | FREE Webinar
Join this webinar to hear from Dr Emilie Desfosses as she shares insights into how in vitro and in silico methods can support more informed, human-relevant safety decisions -especially as ethical and regulatory changes continue to reshape preclinical research.
What you’ll learn:
- Approaches for prioritizing follow-up studies and refining risk mitigation strategies
- How to interpret hit profiles from binding and functional assays
- Strategies for identifying organ systems at risk based on target activity modulation
- How to use visualization tools to assess safety margins and compare compound profiles
Register Now – It’s Free!
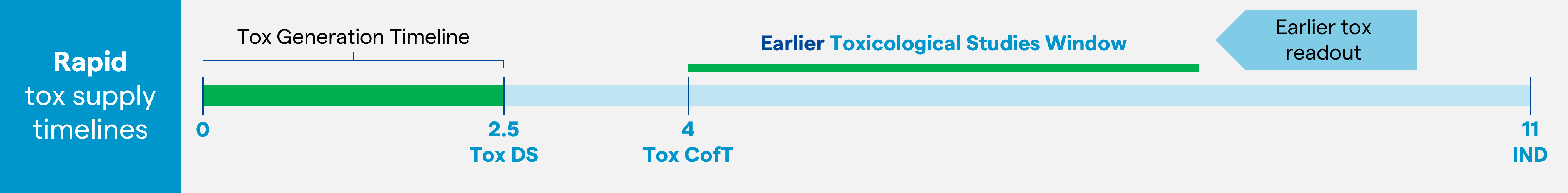
Relatively recently, the concept of non-animal studies for toxicology data generation has been discussed and Food and Drug Administration (FDA) guidance in this direction was issued in December 2022.1 The concept of in-silico models as surrogate for animal models is also an option for drug developers to generate initial safety data. However, navigating the relatively new regulatory landscape makes this a challenging approach and a more risk adverse pathway is generally sought, hence Tox DS material remains the preferred route to early Tox readout. To this end, a ‘pool for Tox’ approach is positioning as a more achievable strategy, where stable transfectant cell pools are expanded prior to selection of the lead cell line. This enables expedited delivery of representative Tox material, particularly for standard mAbs, allowing early initiation of IND-enabling toxicology studies (two to three months earlier compared with a more standard approach using clonal material for Tox; Figure 1).
Figure 1: Addressing the bottleneck to Tox supply: an early Tox supply allows more time to complete the toxicology studies without impacting IND submission. This image is used as an illustrative example.


Of course, simply taking stable pool expansion for toxicology material supply does not address all challenges and is not a single solution in itself. The picture is far more complex. For example, using a pilot-grade ‘pool for tox’ approach for toxicology studies may present a risk to drug developers because companies must understand how this material’s characteristics are likely to differ from later GMP drug-substance material, which is produced using the lead clone selected and will be used in the first-in-human (FIH) studies. Fortunately, applying prior process and manufacturing knowledge, and using process platform technologies, CDMOs and drug developer players are leveraging in-house comparability data to validate this approach and optimise the path to IND without increased development risks.
Using ‘pool for tox’ is a reliable approach and we have seen success for toxicology studies and for FIH studies. 2, 3 In fact, non-clonal pool derived tox material can be seen as beneficial for determining the safety of the material compared to the clonal material. For example, tox material from a pool of clones will contain a potentially broader profile of product quality attributes (eg glycan profile, high molecular weight species, etc) in addition to potentially slightly higher relative amounts of process impurities which can resemble the worst-case scenario during the toxicology testing. Although these differences are in general relatively minor or insignificant, with the correct analytical tools, evaluations and process understanding, the product comparability between pool-for-tox strategy and clonal GMP batch can be demonstrated.
Technology enablers: increasing speed while decreasing risk
The path to expedited Tox material supply requires a fine balance between speed and risk. Managing this balance requires access to extensive development process expertise and a well-defined risk appetite and tolerance by biopharmaceutical companies, but also an efficient cross-functional collaboration and a reliable material generation with expedited review processes (Figure 2).
Figure 2: Securing a fast-track Tox development requires access to extensive development process knowledge and efficient cross-functional collaboration to reach a fine balance between speed and managing risks

Another requirement for earlier Tox material supply is in its formulation: companies with substantial formulation experience and in-house data might take a platform approach and base a formulation on similar molecules and stability data sets, but this may prove challenging to early-stage and smaller biotech companies, where such companies, that lack this wealth of in-house formulation data. This approach also requires an early identification of molecular liabilities and rheological properties, generally using research-grade material supply, in conjunction with in-silico modelling to de-risk drug formulation. However, information would be required on whether the nominated formulation would be sufficiently stable to support the rapid formulation approach and subsequent comparison to the clinical supply. Therefore, there would be almost no appetite for trying to justify a change in formulation between Tox and clinical supplies.
Accelerating timelines to toxicology work also means that many development steps are carried out in parallel and rely on previous and simultaneous stages for an optimal outcome. All these activities, in addition to provision of the actual Tox batch, make significant material demands on the drug substance supply. Historically, this was a significant challenge given low titers in pools compared to the subsequent lead cell line. Advances in new technologies such as engineer transposase-based semi-targeted transgene integration, which integrates the genes of interest into highly active regions of the host genome, have enabled the generation of consistently and predictably high-yielding cell lines, as well as, supporting rapid stable pool generation.
New bioprocessing trends have emerged to cope with these needs. For example, advances in media and feeds have contributed to higher cell protein expression by optimising nutrient availability and reducing accumulation of waste products within a culture. Novel vector design and synthetic promoters have been designed to maximise productivity and enhance gene expression stability. Similarly, scaled‑down high-throughput screening (HTS) and high-resolution analytical methods tools have been pivotal in rapidly evaluating a range of conditions eg, formulations and purification chemistries, allowing better study design for optimal selection of process parameters to advance into confirmation scale-up and robustness testing, while minimising sample requirements and reducing time and costs.
Biologics development of the future
In the near future, accelerated CMC timelines with rapid Tox material supply will be a routine approach not just for standard mAb molecules, but also for complex molecules such as bi-specific antibodies (bsAbs) and non-Fc molecules (non-Protein A binders). Currently, this acceleration can be achieved by eliminating verification testing, simplifying comparability studies and streamlining operations. However, as the biopharmaceutical industry evolves, the role of digital biomanufacturing will deliver evidence-based processes that will support in-silico process design and experimental validation for drug development.4 Moreover, as the push towards smart process analytical technology (PAT) grows stronger, the role of real-time process monitoring and control and ultimately real-time release will ensure better products at reduced speed and therefore cost.
Conclusion
Advances in HTS and analytical testing, as well as vector design and cell line creation and selection, are all contributing to shifting the focus of rapid timelines to toxicological testing as this is becoming the bottleneck for many programmes. As we look to the future, it is likely the same considerations will apply to more complex protein therapeutics and non-platform processes in the race to regulatory approval.
Aligned with speed to clinic, the technological advances discussed here are being complemented by a gradually changing regulatory landscape; not only as mentioned above in terms of migration to non-animal Tox models but also with respect to the relatively recent publication of draft guidance for industry by the FDA on their proposed Platform Technology Designation Program. This describes an approach where developers can apply for platform status for a specific technology and thereby use this to both accelerate drug development and reduce the review time.
As new technologies push timelines along with regulatory evolution and as we move into an era with increasing in-silico reliability and the use of artificial intelligence in identifying new drug targets, the provision of Tox material will become an increasingly hot topic across the biopharma sector.
References:
1 Wadman M. FDA no longer has to require animal testing for new drugs. Science. 2023 Jan 13;379(6628):127-128
2 Tan KW, et al. Rapidly accelerated development of neutralizing COVID-19 antibodies by reducing cell line and CMC development timelines. Biotechnol Bioeng. 2022 Dec 8:10.1002/bit.28302
3 Broly H, Souquet J, Beck A. Effects of the COVID-19 pandemic: new approaches for accelerated delivery of gene to first-in-human CMC data for recombinant proteins. MAbs. 2023 Jan-Dec;15(1):2220150.
4 Berrie J, Donninger R. How Emerging Biotech can benefit. Network Pharma Magazine. 2024:24-26
About the authors
Alejandro Fernandez Martell, Global Principal Scientist, Lonza


Alejandro holds a BEng in biotechnology from the IPN, Mexico, and a PhD in chemical and biological engineering from the University of Sheffield, UK.
James Berrie, Technical Director, Global Process Development, Lonza


James’ expertise involves the application of speed enabling technologies and regulatory updates to advise new and existing customers on approaches to efficient CMC strategies.
James holds a BSc in biochemistry from Queen Mary, University of London and a PhD in biochemistry from St Bartholomew’s & The Royal London School of Medicine and Dentistry.
Related topics
Antibodies, Clinical Trials, Drug Development, Therapeutics, Toxicology
Related organisations
Lonza