Combining precision bispecific antibodies with targeted radiotherapy
Posted: 8 October 2024 | Alexander Schinagl (OncoOne) | No comments yet
Radioimmunotherapy (RIT) is a promising cancer treatment gaining recognition in precision oncology. It utilises radiolabelled antibodies to deliver targeted radiation to tumours, with success in hematologic cancers. However, its use in solid tumours has been limited by off-target radiation and toxicity, with few clinical trials advancing beyond phase II. Alexander Schinagl, cofounder of OncoOne, explains how the company’s PreTarg-it® platform uses pretargeted RIT, offering a novel approach improving tolerability and effectiveness to optimise therapeutic responses in solid tumours.

In the PreTarg-it® system, why is the three-to-five-day interval between ON105 administration and the delivery of the radiolabelled DOTA-di-HSG peptide critical for maximising therapeutic efficacy and minimising off-target effects?
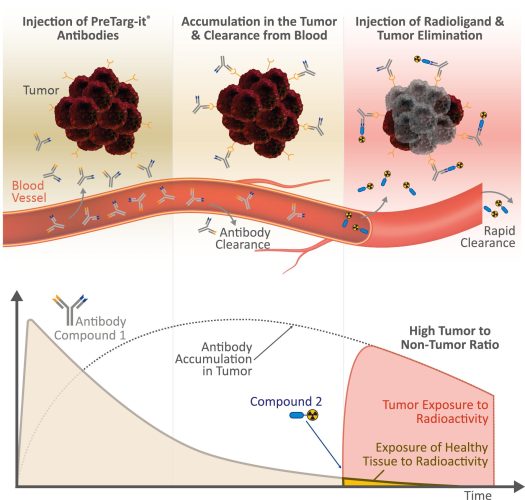
Our PreTarg-it® platform enhances the efficacy of radioimmunotherapy (RIT) while reducing off-target radiation by employing a pretargeted approach. This involves administering our bispecific antibody, ON1051 which targets the tumour-specific antigen, oxMIF 2–4 with one arm, followed by a radioligand, 177Lu-IMP288 (a DOTA-di-HSG peptide), which binds to the second arm of ON105. The three-to-five-day delay between administrations allows ON105 to accumulate in the tumour and clear from the bloodstream and healthy tissue. Due to its small size, the radioligand penetrates solid tumours efficiently and binds to ON105 inside the tumour, while unbound molecules are rapidly excreted. By timing the radioligand administration to follow the clearance of ON105, radiation is concentrated in the tumour, minimising systemic exposure and increasing the therapeutic index. This strategy supports the safe escalation of radiation doses, with most radioactivity being confined to the tumour or quickly eliminated.
In terms of radiolabel stability and half-life, what advantages does 177Lu-DOTA-di-HSG offer for pre-targeted RIT, and how does this compare to other radionuclides or chelators used in similar therapeutic regimens?
DOTA’s strong binding affinity to metal ions such as ¹⁷⁷Lu and other medical radioisotopes used for therapeutic or diagnostic purposes, along with its demonstrated safety profile in approved radioligand therapies, has made it the most widely used chelator in pretargeting strategies to date. With a half-life of approximately 6.7 days, ¹⁷⁷Lu aligns well with the tumour retention time of the bispecific antibody, providing an optimal window for effective tumour targeting and the clearance of non-targeted radioligand molecules. Although DOTA has been applied as a versatile and effective chelator for alpha, beta, and gamma emitters, our PreTarg-it® platform provides additional flexibility. DOTA can be readily substituted with other chelators that efficiently bind to various radionuclides with therapeutic potential, such as 67Cu, 90Y, 213Bi or 255Ac. These radionuclides, paired with their respective chelators, may offer distinct advantages for targeting different tumour types.
How does PreTarg-it® address the potential for radio resistance or immune evasion mechanisms that solid tumours often exhibit?
Our PreTarg-it® platform is designed to tackle radioresistance in solid tumours by enhancing the precision of radiation delivery. PreTarg-it® utilises bispecific antibodies that specifically target tumour-associated antigens like oxMIF (with ON105), MSLN, HER2, or FRα. This ensures that high doses of radiation are delivered directly to cancer cells while sparing healthy tissues, which allows for higher, local radiation doses to overcome resistant cells. The PreTarg-it® platform is highly flexible and can employ both alpha emitters (eg, 225Ac, 213Bi, and 212Pb) which are ideal for eradicating small clusters of cancer cells, and beta emitters (eg, 177Lu, 90Y, and 67Cu), which are capable of deeply penetrating cancer tissue to overcome radioresistance in challenging tumour microenvironments including dense stroma or poorly vascularised areas. As PreTarg-it® does not rely on the immune system to deliver cytotoxicity directly into the tumour, it can also circumvent the typical challenges associated with immune evasion mechanisms.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
Given the high survival rates observed in murine models with CT26 and CFPAC-1 tumours, what are the anticipated hurdles in translating the PreTarg-it® approach to clinical settings?
Based on our experience and data, the key to success lies in the sophisticated design of the bispecific antibody. The OncoOne team is engineering antibodies to optimise pharmacokinetics, biodistribution, tumour retention, and target specificity. PreTarg-it® is being developed to target tumour markers in challenging cancers, such as pancreatic cancer and our next goal will be to advance our platform toward clinical testing in order to bring an innovative treatment method to patients with limited options.
Selecting IMP288 as our radioligand of choice will enable the use of positron emitters for PET scans and patient stratification, as well as the application of valuable therapeutic medical radioisotopes including ¹⁷⁷Lu. The use of IMP288 is a key advantage, as it has already been clinically evaluated, demonstrating specific tumour penetration and rapid elimination through renal excretion.5–9 The pretargeting approach expands patient access to RIT at specialised centres by allowing the bispecific antibody and the radioisotope to be delivered separately. This enables more flexible scheduling and simplifies logistics, making treatment more accessible.
Figures and Figure Legends
Figure 1: The PreTarg-it® platform consists of two components: Compound 1, a bispecific antibody that targets tumour markers, and Compound 2, a radioligand that binds to the bispecific antibody. The platform is highly flexible as Compound 1 can be easily modified to target different cancer markers (“Target X”), while Compound 2 can be adapted with various radioactive payloads (“Payload Y”) for both diagnostic and therapeutic purposes. Figure created by biolution GmbH
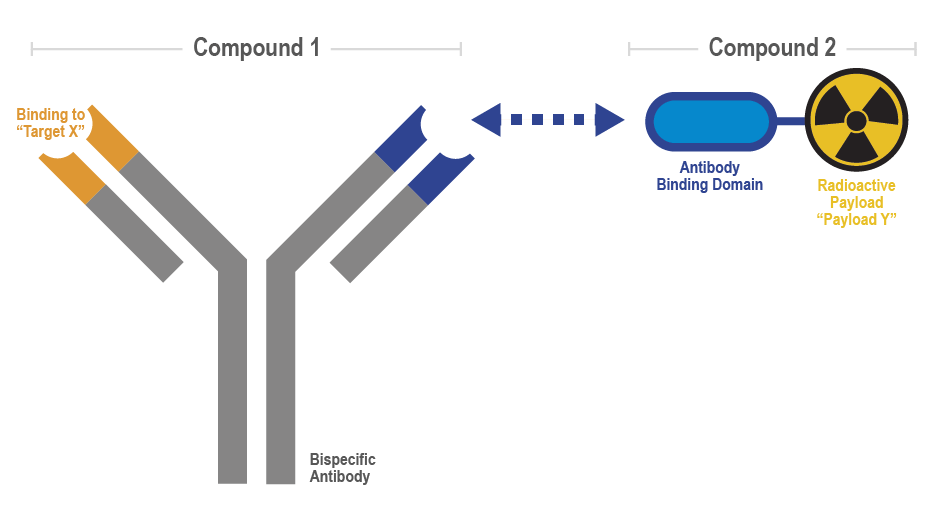

Figure 2: The PreTarg-it® platform utilises a pretargeted RIT approach that consists of three steps: 1. Administering a bispecific antibody with one arm targeting a specific tumour marker; 2. Allowing the antibody to clear from the bloodstream and concentrate within the tumour; 3. Delivering a radioligand that binds to the antibody’s other arm. Any unbound radioligand is quickly eliminated via the kidneys, reducing systemic exposure and enhancing the therapeutic index. Figure created by biolution GmbH.

References
1 Puchol Tarazona AA, Schinagl A, Mirkina I, Rossmueller G, Kerschbaumer RJ, Bachmann F, Thiele M (2024) Pretargeted Radioimmunotherapy with the Novel Anti-oxMIF/HSG Bispecific Antibody ON105 Results in Significant Tumor Regression in Murine Models of Cancer. Mol Cancer Ther 23:1219-1229
2 Rossmueller G, Mirkina I, Maurer B, Hoeld V, Mayer J, Thiele M, et al. Preclinical Evaluation of ON203, A Novel Bioengineered mAb Targeting Oxidized Macrophage Migration Inhibitory Factor as an Anticancer Therapeutic. Molecular Cancer Therapeutics. 2023 Apr 17;22(5):555–69.
3 Schinagl A, Thiele M, Douillard P, Völkel D, Kenner L, Kazemi Z, et al. Oxidized macrophage migration inhibitory factor is a potential new tissue marker and drug target in cancer. Oncotarget. 2016 Sep 12;7(45):73486–96.
4 Kerschbaumer R, Rieger M, Völkel D, Didier Le Roy, Roger T, Jurate Garbaraviciene, et al. Neutralization of Macrophage Migration Inhibitory Factor (MIF) by Fully Human Antibodies Correlates with Their Specificity for the β-Sheet Structure of MIF. Journal of Biological Chemistry. 2012 Mar 2;287(10):7446–55.
5 Touchefeu Y, Bailly C, Frampas E, Eugène T, Rousseau C, Bourgeois M, et al. Promising clinical performance of pretargeted immuno-PET with anti-CEA bispecific antibody and gallium-68-labelled IMP-288 peptide for imaging colorectal cancer metastases: a pilot study. European Journal of Nuclear Medicine and Molecular Imaging. 2020 Aug 21;48(3):874–82.
6 Rousseau C, Goldenberg DM, Colombié M, Sébille JC, Meingan P, Ferrer L, et al. Initial Clinical Results of a Novel Immuno-PET Theranostic Probe in Human Epidermal Growth Factor Receptor 2–Negative Breast Cancer. Journal of Nuclear Medicine. 2020 Aug 1;61(8):1205–11.
7 Schoffelen R, Boerman OC, Goldenberg DM, Sharkey RM, van Herpen CML, Franssen GM, et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: first clinical results. British Journal of Cancer. 2013 Aug 20;109(4):934–42.
8 Bodet-Milin C, Faivre-Chauvet A, Carlier T, Rauscher A, Bourgeois M, Cerato E, et al. Immuno-PET Using Anticarcinoembryonic Antigen Bispecific Antibody and 68Ga-Labeled Peptide in Metastatic Medullary Thyroid Carcinoma: Clinical Optimization of the Pretargeting Parameters in a First-in-Human Trial. Journal of Nuclear Medicine. 2016 May 26;57(10):1505–11.
9 Bodet-Milin C, Ferrer L, Rauscher A, Masson D, Rbah-Vidal L, Faivre-Chauvet A, et al. Pharmacokinetics and Dosimetry Studies for Optimization of Pretargeted Radioimmunotherapy in CEA-Expressing Advanced Lung Cancer Patients. Frontiers in Medicine [Internet]. 2015 Nov 27 [cited 2024 Sep 27];2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4661432/
About the author
Alexander Schinagl, co-founder and Chief Technology Officer at OncoOne


Alexander Schinagl, co-founder and Chief Technology Officer at OncoOne, has over 15 years of experience in the biotechnology and pharmaceutical industries, where he has acquired a profound background in antibody engineering, drug discovery, development, and translational research. He received his PhD in biotechnology through an industry collaboration with Baxter BioScience and Baxalta from the University of Natural Resources and Life Sciences (BOKU) in Vienna. Before joining OncoOne, he led discovery projects involving antibodies and antibody-drug conjugates in oncology, inflammation, and hematologic disorders at Baxter BioScience, Baxalta, and Shire.
Related topics
Antibodies, Cancer research, Drug Delivery, Drug Development, Oncology, Therapeutics
Related conditions
Cancer, solid tumours
Related organisations
OncoOne
Related people
Alexander Schinagl (OncoOne)