Beyond ELISA: the future of biomarker validation
Posted: 27 November 2024 | Prasad Lakshmanan Selvaraj (Sannova) | No comments yet
The era of precision medicine demands more rigorous biomarker validation methods. While ELISA has long been the gold standard, advanced technologies such as LC-MS/MS and Meso Scale Discovery (MSD) offer superior precision, sensitivity and efficiency. Here, Prasad Lakshmanan Selvaraj, Director of Technical Review at Sannova, explains that by embracing these cutting-edge approaches, drug developers can accelerate biomarker qualification, reduce costs, and stay ahead of evolving regulatory standards.

Introduction
Biomarkers are becoming increasingly essential in drug development and clinical practice, driving the need for more precise validation methods. As the demand for personalised treatments grows, regulatory bodies are adapting their standards to support advanced techniques that go beyond traditional approaches. For example, the European Medicines Agency (EMA), in its Regulatory Science Strategy to 2025, has highlighted the critical role of biomarker discovery, qualification and utilisation in accelerating precision medicine.1
The journey to qualifying biomarkers for clinical and regulatory use is fraught with challenges, leading to a remarkably low success rate. For example, only about 0.1 percent of potentially clinically relevant cancer biomarkers described in literature progress to routine clinical use.2 The biomarker development pipeline involves multiple stages, including discovery, analytical validation, clinical validation and regulatory qualification.
only about 0.1 percent of potentially clinically relevant cancer biomarkers described in literature progress to routine clinical use
Many candidates fail to pass through all these stages due to a lack of reproducibility, correlation with clinical outcomes or sufficient validation. The regulatory qualification process, particularly by agencies like the US Food and Drug Administration (FDA), requires robust evidence of both the validity and utility of a biomarker, adding to the complexity. These obstacles highlight the need for advanced tools and approaches to improve the success rate in biomarker development.
Advanced techniques like liquid chromatography tandem mass spectrometry (LC-MS/MS) and Meso Scale Discovery (MSD) provide the kind of comprehensive and reliable data increasingly favoured by regulators, yet many researchers continue to rely exclusively on enzyme-linked immunosorbent assay (ELISA). This article highlights the benefits of leveraging advanced biomarker analysis methods, and how they ultimately save drug developers time and money by getting one step ahead of regulatory requirements.
The evolving landscape of biomarker validation
ELISA is widely regarded as the gold standard for biomarker validation and clinical diagnostics owing to its exceptional specificity, sensitivity and ability to quantify proteins in biological samples. Known for its versatility and robustness, ELISA enables relatively high-throughput analysis and is commonly used in confirmatory studies.
However, ELISA does have certain limitations. For example, its performance is highly dependent on the quality of antibodies and expertise of the operator. Additionally, ELISA has a relatively narrow dynamic range compared to some multiplexed immunoassays3,4 and developing a new ELISA assay can also be both costly and time-consuming. Finally, its application in urine samples presents additional challenges compared to serum.5
Alzheimer’s disease: driving advancements with precision medicine
In contrast, advanced technologies like MSD and LC-MS/MS offer enhanced precision and sensitivity for biomarker analysis. MSD, utilising electrochemiluminescence (ECL) detection, provides up to 100 times greater sensitivity than traditional ELISA, enabling the detection of lower abundance proteins and a broader dynamic range. LC-MS/MS also surpasses ELISA in sensitivity, making it a useful technique for detecting low-abundance species.
MSD also provides advanced platforms that support researchers in biomarker development, offering tools that streamline both discovery and validation processes. One key offering is MSD’s U-PLEX multiplexed immunoassay platform, which allows researchers to design custom biomarker panels and measure multiple analytes simultaneously within a single sample.6
By enabling the simultaneous analysis of multiple biomarkers in small sample volumes, MSD’s assays enhance efficiency in biomarker research, especially when dealing with complex diseases or therapeutic responses. LC-MS/MS goes even further, allowing the analysis of hundreds to thousands of proteins in a single run.5
MSD’s assays enhance efficiency in biomarker research, especially when dealing with complex diseases or therapeutic responses
The success of a biomarker in clinical and regulatory settings hinges on several factors, including analytical and clinical validity. Analytical validity refers to the robustness and reproducibility of the measurement. Clinical validity, meanwhile, depends on the consistent correlation of the biomarker with clinical outcomes,7 which is often a key hurdle. Furthermore, achieving success requires ‘fit-for-purpose’ validation, where the level of validation is tailored to the intended clinical use of the biomarker.
Cost is a critical consideration in biomarker research, especially when comparing traditional methods like ELISA with more advanced technologies. For example, measuring four inflammatory biomarkers (IL-1β, IL-6, TNF-α and IFN-γ) using individual ELISAs costs approximately $61.53 per sample.8 In contrast, using MSD’s multiplex assay reduces the cost to $19.20 per sample. This represents a saving of $42.33 per sample when opting for MSD’s technology, highlighting both its economic and operational efficiency. By reducing costs without sacrificing quality, MSD’s multiplexing technology offers a compelling alternative for laboratories facing budgetary constraints.
Advanced techniques like MSD and LC-MS/MS offer unmatched precision and sensitivity at a fraction of the cost. Given this, what’s holding drug developers back?
Complications
The primary hurdles are access and cost. However, outsourcing has emerged as a game changer, addressing both issues effectively. By outsourcing, drug developers can harness the power of these advanced techniques without the upfront investment and infrastructure costs.
Additionally, some may worry that regulators prefer traditional methods. This is a misconception. Agencies like the FDA and EMA welcome comprehensive information from the outset, as it facilitates a smoother and more efficient review process. Having this information on the table during the initial pre-investigational new drug (pre-IND) meeting provides drug developers with a significant advantage, enabling them to stay ahead of evolving regulatory demands.
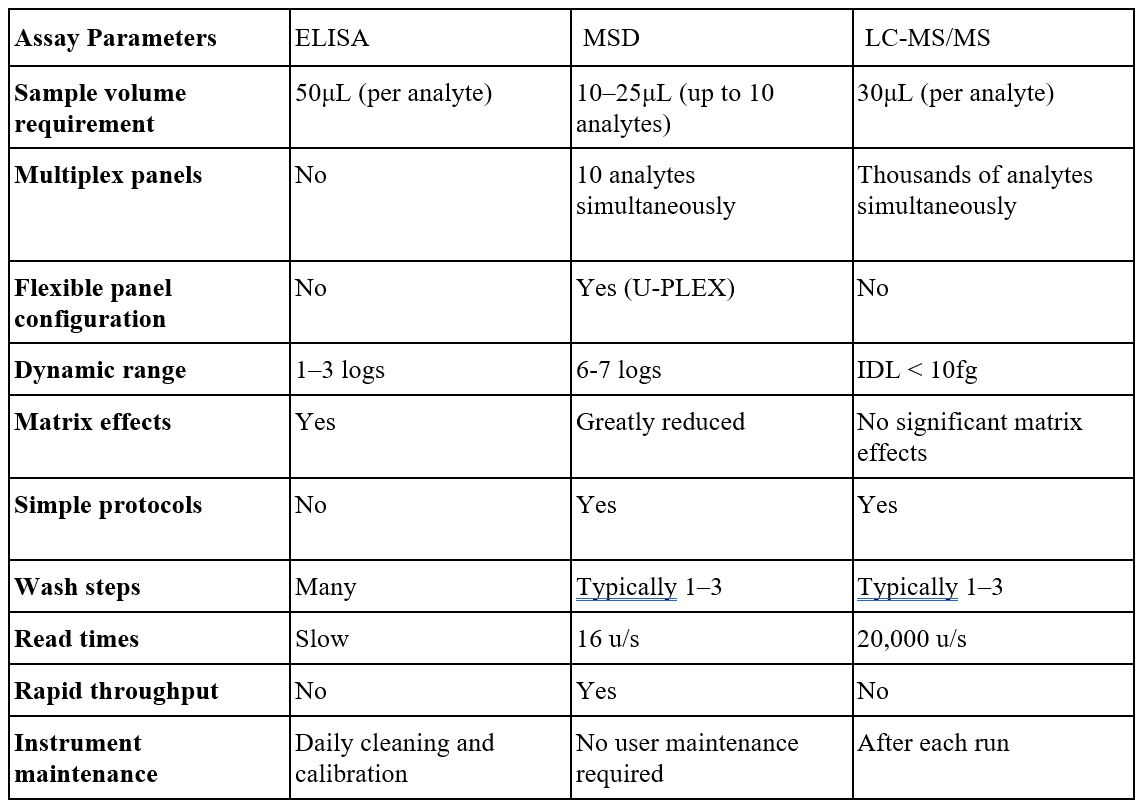

Table 1: Side-by-side comparison of ELISA, MSD and LC MS/MS assay parameters.
Regulatory overview
Regulatory requirements for biomarker validation are evolving to address the growing need for precision and relevance. Both the FDA and EMA now advocate for a tailored approach to biomarker validation,9,10 emphasising that it should be aligned with the specific intended use of the biomarker rather than relying on a one-size-fits-all method.
To ensure robust and reliable results, regulators are demanding more comprehensive validation data, including enhanced analytical validity, such as accuracy and precision of assays. This means using independent sample sets and cross-validation techniques to strengthen the evidence supporting biomarker effectiveness.
As a result, the FDA and EMA have introduced formal biomarker qualification processes. These processes offer a regulatory stamp of approval, validating the biomarker’s suitability for use in drug development and ensuring it meets the highest standards.
The extensive review of the EMA biomarker qualification procedure by Bakker et al.10 reveals a critical insight: a staggering 77 percent of biomarker challenges were linked to assay validity. The review also highlighted frequent issues that led to rejection, including problems with specificity, sensitivity, detection thresholds and reproducibility. This underscores an urgent need for methodological precision and rigorous adherence to validation standards to overcome these challenges and ensure the success of biomarker qualification.
The future of biomarker validation
In recent years, there has been a significant increase in the outsourcing of biomarker testing to contract research organisations (CROs). The global biomarker discovery outsourcing service market was estimated at $2.7 billion in 2016 and continues to grow.11 Pharmaceutical companies are increasingly outsourcing due to resource and capacity constraints, the need for specialised expertise, and the flexibility outsourcing offers in project management.
CROs provide access to cutting-edge technologies and support regulatory compliance, making them an attractive option for companies looking to streamline their biomarker development process. This trend reflects the growing complexity of biomarker testing and the need for external support in achieving successful qualification.
Given the unparalleled specificity and sensitivity of advanced methods like MSD and LC-MS/MS, their superior detection limits, and their expansive dynamic ranges, it’s no wonder that regulators are increasingly favouring these techniques. Their freedom from matrix effects further boosts their reliability and confidence in results.
Drug developers are also eagerly embracing these innovations, fuelled by the growing trend of outsourcing and the understanding that investing in high-quality methods early on pays dividends by reducing wasted time and resources in later stages. As precision medicines, including RNA interference (RNAi) and oligonucleotide therapies, become more prominent, the importance of advanced techniques in streamlining biomarker validation and expediting the regulatory review process becomes even clearer. Embracing these cutting-edge methods is not just a strategic choice but a crucial step towards staying at the forefront of drug development.
About the author
Prasad Lakshmanan Selvaraj, Director of Technical Review, Sannova
Prasad has 19 years of experience in the bioanalytical field catering to biotech and biopharma drug development projects. Since 2005, he has played a vital role in ensuring that laboratory data meets rigorous quality standards and complies with industry regulations. Being in the cross-section of changing regulations and advancing technologies, Prasad leads the charge on adapting new methodologies in the lab and driving capability expansion as well as process improvements.
References
- Hendrikse NM, Llinares Garcia J, Vetter T, et al. Biomarkers in Medicines Development—From Discovery to Regulatory Qualification and Beyond. Frontiers in Medicine. 2022 Apr 26;9:878942.
- Kern SE. Why your new cancer biomarker may never work: recurrent patterns and remarkable diversity in biomarker failures. Cancer research. 2012 Dec 1;72(23):6097-101.
- Meso Scale Discovery – MSD – Pacific BioLabs [Internet]. Pacific BioLabs. 2023 [cited 2024 Oct 22]. Available from: https://pacificbiolabs.com/meso-scale-discovery-msd/
- Köhler K, Seitz H. Validation Processes of Protein Biomarkers in Serum—A Cross Platform Comparison. Sensors. 2012 Sep 18;12(9):12710–28.
- Muntel J, Gandhi T, Verbeke L, et al. Surpassing 10000 identified and quantified proteins in a single run by optimizing current LC-MS instrumentation and data analysis strategy. Molecular omics. 2019;15(5):348-60.
- Bastarache JA, Koyama T, Wickersham NE, Ware LB. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. Journal of immunological methods. 2014 Jun 1;408:13–23.
- Bossuyt PM. Clinical validity: defining biomarker performance. Scandinavian Journal of Clinical and Laboratory Investigation. 2010 Jan 1;70(sup242):46-52.
- Leng SX, McElhaney JE, Walston JD, et al. ELISA and Multiplex Technologies for Cytokine Measurement in Inflammation and Aging Research. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008 Aug 1;63(8):879–84.
- FDA. Biomarker Qualification: Evidentiary Framework [Internet]. U.S. Food and Drug Administration. 2018. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/biomarker-qualification-evidentiary-framework
- Bakker E, Hendrikse NM, Ehmann F, et al. Biomarker Qualification at the European Medicines Agency: A Review of Biomarker Qualification Procedures From 2008 to 2020. Clinical Pharmacology & Therapeutics. 2022 Mar 5;112(1):69–80.
- Holt R. Outsourcing Biomarkers in Clinical Trials: Advantages and Disadvantages. Handbook of Biomarkers and Precision Medicine, pp. 174-177. Chapman and Hall/CRC, 2019.
Related topics
Analysis, Analytical Techniques, Assays, Biomarkers, Immunoassays, Screening, Technology
Related organisations
European Medicines Agency (EMA), US Food and Drug Administration (FDA)