New insights into the role of viral capsids in gene therapy safety
Posted: 5 December 2024 | Dr Chelsea B Pratt | No comments yet
Viral capsid assembly and quality are key factors in gene therapy success and safety. Here, Dr Chelsea Pratt explores how cutting-edge tools are enabling scientists to tackle these challenges and improve patient outcomes.

Viral vectors have been crucial in transforming the gene therapy landscape due to their natural ability to infect cells. Among them, adeno-associated viruses (AAVs) stand out due to their small size, high efficiency in gene delivery, low immunogenicity, and potential for customisable tissue targeting, making them the leading tool in gene expression research.1 In 2017, the US Food and Drug Administration (FDA) approved the first AAV-based gene replacement therapy (Luxturna), for Leber congenital amaurosis type 2.2 Since then, the FDA has approved four more AAV-based gene therapies—Zolgensma, Hemgenix, Elevidys and Rocktavian—for treating various diseases. The large number of ongoing clinical trials of recombinant AAV (rAAV)-based therapies highlights their promise for addressing a wide variety of human genetic disorders. 2
Structure and function of AAV capsids in gene therapy
Wild-type AAV is composed of a protein shell (capsid) that contains a single-stranded DNA genome encoding proteins involved in viral replication, structure and assembly.2 The capsid is composed of three structural proteins of differing sizes, and the variability in these proteins among viral isolates is used to characterise AAV serotypes, contributing to their broad tissue tropism. At least 12 naturally occurring serotypes and over 1,000 variants have been discovered over the past two decades. Moreover, some serotypes possess inherent properties that promote cellular entry and transgene expression. Therefore, vectors based on these serotypes may enable targeted gene delivery and optimal transduction efficiency.
rAAV vectors retain capsid sequences but replace viral genes with transgene expression cassettes, making them replication-incompetent.2,3 Engineering both the capsid and DNA cargo improves the safety and efficacy of rAAV vectors and expands their clinical applications. However, the production process of fully assembled rAAV particles generates by-products and requires thorough characterisation to ensure efficacy while minimising the potential for immunogenicity and off-target effects. These production challenges should be met with precise and sensitive tools that can prevent roadblocks during vector development for new therapies.
Challenges of viral capsids in gene therapy efficiency
A widely used production method of rAAV vectors requires co-transfection of host cell lines with three plasmids, including one carrying the transgene of interest, one carrying the capsid and replication sequences, and a helper virus to circumvent the inability of rAAV to self-amplify.2 However, utilisation of biological systems introduces variability and by-products, as well as a challenge unique to AAV production: the assembly of full capsids with correctly packaged DNA, partially filled capsids containing partial vector and/or host cell DNA, and empty capsids. Determining the ratio of full, partially filled, and empty capsids is considered a Critical Quality Attribute (CQA) for AAV gene therapy products, and is a factor that must be monitored and controlled, as higher empty capsid contents in gene therapies poses risks of triggering an immune response and adverse events in the clinic (Figure 1).4-6 In addition, a substantial presence of partially filled or empty capsids can negatively impact transduction efficiency and thus reduce the efficacy of AAV products. As a result, there is a need for validated AAV testing solutions to meet quality standards as well as compliance and regulatory requirements.
Deviations in the ratios of the three structural capsid proteins (VP1-3) indicate improper assembly of AAV capsids. The molar ratio of VP1:VP2:VP3 in rAAV capsids should fall around 1:1:10, similar to most wild-type AAV capsids,7 to ensure stability and potency.1 Further, confirming serotype identity is necessary to achieve specific tissue targeting. While the separation of capsid proteins by techniques like SDS-PAGE and western blotting enables the identification of VP subtypes, they are time-intensive, multi-step processes. Alternative workflows are needed to support high-throughput analysis, such as that required during the production of gene therapies.
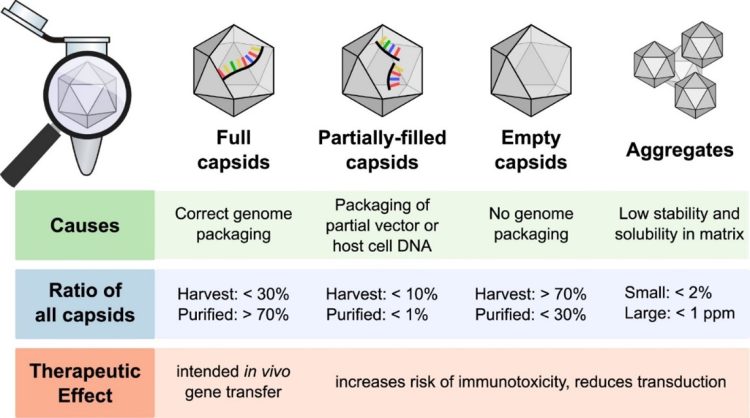

Figure 1. Major capsid types formed during rAAV production and impact of therapeutic outcomes.6 (CC BY 4)
Overcoming traditional analytical barriers in capsid ratio determination
While there are numerous analytical technologies available on the market for testing rAAV capsid ratios, none of the current methods are endorsed by regulatory agencies for large-scale product manufacturing. Many commonly used methods, such as Analytical Ultracentrifugation (AUC) and Enzyme-Linked Immunosorbent Assay (ELISA, used in combination with qPCR) have longstanding use in the identification of full, partially filled, and empty capsids, but they have their own limitations. These include constraints in throughput, accuracy, precision, protocol length and/or the amount of sample required for analysis.6 Further, rAAV capsid ratios should be measured at various stages of production, as they vary when particles are harvested from cell culture as well as after purification steps. Therefore, the development of a novel method that allows rapid, high-throughput analysis of rAAV capsid ratios with high accuracy and precision is warranted.
Recently, development of a droplet-based digital PCR approach (eg, ddPCR), has allowed for fast and robust evaluation of viral capsid ratios with high precision and accuracy. By enabling the absolute quantification of proteins and viral genomes within droplets, ddPCR is a versatile technology that can be employed across different complexities of biological samples, ranging from crude lysates to purified samples. Additionally, ddPCR analysis requires low sample input, enables simultaneous measurement of protein and genome contents, and maintains speed and compatibility across various manufacturing steps.
Overall, measurement of empty-full capsid ratios via ddPCR is a fully validated solution for quality control during rAAV production, offering comparable precision to AUC and higher accuracy to ELISA + qPCR while supporting compliance and regulatory considerations (Figure 2)8. In comparison to ddPCR technology, traditional methods such as AUC cannot differentiate when contaminants are encapsulated within capsids as it separates particles based on their size, weight and density. Thus, ddPCR-based assays ensure comprehensive characterisation of capsids by providing information on genome titer, capsid titer, and the percentage of full capsids – determined using a single test without external standard curves.
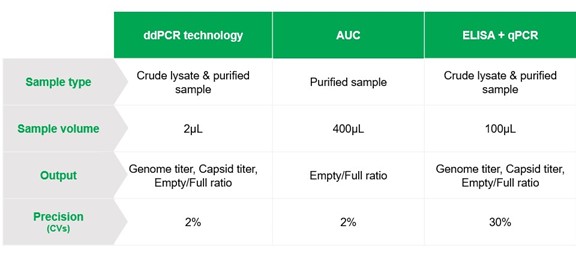

Figure 2. Comparative analysis of key capabilities of ddPCR, AUC and ELISA + qPCR for rAAV production quality control.
Looking ahead
While AAV-based gene therapies hold promise in treating a range of genetic disorders, strict quality control of empty and partially filled viral capsids produced during manufacturing is critical to maintain the safety and efficacy of rAAV products. Absolute quantification of both viral proteins and genome by ddPCR is an innovative methodology poised to advance gene therapy research, providing rapid, precise, and accurate analysis of both crude lysates and purified samples. Access to fast, high-throughput tools during the manufacturing process will accelerate the development of therapeutics while adhering to regulations and stringent quality standards.
References
- Pratt CB, Alsarraj MA. Navigating developmental challenges in AAV gene therapy [Internet]. Labcompare. 2024 [cited 2024 Oct 28]. Available from: https://www.labcompare.com/10-Featured-Articles/611315-Navigating-Developmental-Challenges-in-AAV-Gene-Therapy/
- Wang JH, Gessler DJ, Zhan W,et al. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct Target Ther. 2024;9(1):78.
- Brommel CM, Cooney AL, Sinn PL. Adeno-associated virus-based gene therapy for lifelong correction of genetic disease. Hum Gene Ther. 2020;31(17-18):985-95.
- McColl-Carboni A, Dollive S, Laughlin S, et al. Analytical characterization of full, intermediate, and empty AAV capsids. Gene Ther. 2024;31(5-6):285-94.
- Wright JF. Product-related impurities in clinical-grade recombinant AAV vectors: characterization and risk assessment. Biomedicines. 2014;2(1):80-97.
- Gimpel AL, Katsikis G, Sha S, et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol Ther Methods Clin Dev. 2021;20:740-54.
- Van Vliet KM, Blouin V, Brument N, et al. The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol. 2008;437:51-91.
- Yin J, Sepulveda N. Performance comparison of the Vericheck ddPCR empty-full capsid kit and analytical ultracentrifugation (AUC). Bio-Rad, Bulletin 3725 [cited 2024 Oct 28]. Available from: https://www.bio-rad.com/sites/default/files/2024-10/Performance-Comparison-of-Droplet-Digital-PCR-and-Analytical-Ultracentrifugation.pdf
About the author


Dr Chelsea B Pratt is the Biopharma Segment Marketing Manager at Bio-Rad Laboratories. In this role, she works with leaders in the biotech and pharmaceutical industries to understand how Bio-Rad can accelerate drug discovery and development in new therapeutic areas. She is a recognised expert in chromatography and protein purification, and serves as a regular panellist at conferences, seminars, and webinars. Dr Pratt holds a PhD in Biological Chemistry from the University of Texas Southwestern Medical Center.
Related topics
Analytical Techniques, Biologics, Gene Therapy, Genomics, Viral vectors
Related conditions
Duchenne muscular dystrophy, Hemophilia A, Hemophilia B, Leber Congenital Amaurosis Type 2, spinal muscular atrophy (SMA)
Related organisations
Bio-Rad Laboratories
Related people
Dr Chelsea B Pratt