New targeted therapies show promise in lung cancer treatment
Posted: 13 December 2024 | Dr Eleni Lagkadinou (Vice President of Oncology Early Development at AbbVie) | No comments yet
Recent advancements in targeted therapies and immunotherapies are transforming lung cancer treatment. Discover how these innovations are improving outcomes and providing hope for patients.

Lung cancer is the most commonly diagnosed cancer worldwide, representing 12.4 percent of all cases. It is also the leading cause of cancer-related deaths, accounting for 18.7 percent of total cancer fatalities.1 Lung cancer is primarily classified into two types based on histological examination: Non-Small Cell Lung Cancer (NSCLC) and Small Cell Lung Cancer (SCLC). These types differ in their etiology, natural history, and present distinct challenges in disease management. NSCLC, which accounts for about 85 percent of cases, is more prevalent, while SCLC, though less common, is notoriously aggressive and requires faster, more effective therapeutic interventions.2
Unmet needs in lung cancer treatment
Recent decades have seen significant advancements in lung cancer treatment, especially with the introduction of targeted therapies and immunotherapies, which have notably improved survival rates.3,4 However, inter- and intra-tumoural heterogeneity is widespread in lung cancer, leading to multiple mechanisms through which these tumours can and do develop resistance to treatment.5 This has created a significant unmet need for new treatments that either prevent resistance or prolong the effectiveness of initial therapies while maintaining a tolerable safety profile. Moreover, many lung cancer patients have unique genetic profiles for which no effective targeted treatments are currently available.6 These patients typically receive standard care, involving immunotherapy and chemotherapy, which often fail to produce durable responses.3 For these patients, improvements in the currently available biomarker-agnostic treatment approaches are crucial.
Opportunities for precision treatment
The identification of specific oncogenes in lung cancer has accelerated the development of precision medicine, particularly targeted therapies designed to address individual genetic alterations or the overexpression of specific markers, such as mesenchymal-epithelial transition factor (c-MET), trophoblast cell surface antigen 2 (TROP2), and seizure-related homolog protein (SEZ6), among others.7,8 The discovery of these biomarkers provides valuable insights into prognosis and disease progression, while also guiding the clinical development of new targeted immunotherapies. Combined with advances in antibody-drug conjugates (ADCs) and bi-specific antibodies (BsAbs), these developments present a promising opportunity to potentially enhance antitumor activity and survival outcomes for lung cancer patients. Moreover, biomarker-driven strategies allow clinicians to categorise patients based on their biomarker status, enabling more effective personalised treatment plans.
Next-generation approaches
Antibody Drug Conjugates (ADCs)
Lung cancer therapy is enhanced by advancements in ADC technology. These next-generation ADCs incorporate features to improve bioavailability, efficacy, and safety 9, 10, 11, such as:
- Developing humanised or fully human monoclonal antibodies (MAb) and/or partial silencing of Fcγ-regions helps minimise host-mediated immune reactivity, given that antibodies can regulate immune responses through Fc receptor interactions.
- Engineering sites on the antibody to enable specific and consistent attachment of the cytotoxic drug to achieve an optimal drug-to-antibody ratio (DAR).
- The development of more hydrophilic linkers helps maintain the structural and functional integrity of the antibody in the final conjugate. Additionally, newer-generation linkers enable more specific and stable payload attachment, potentially improving therapeutic indices and safety.
- Innovations in payload substances, such as topoisomerase inhibitors (topo-1i), and next-generation DNA- damaging agents to replace older, more toxic agents. These can include anti-tubulin compounds, potentially reducing side effects and improving quality of life.12
By utilising these advancements, significant progress has been made in targeting alterations in c-MET, which is implicated in up to 60 percent of NSCLC cases and is associated with a poor prognosis.7 Recent clinical studies highlight promising developments in c-MET-targeting ADCs for NSCLC.13 These include reports of antitumour activity with a c-MET-directed ADC in patients with c-MET-overexpressing NSCLC, as well as in cohorts of patients with concurrent epidermal growth factor receptor (EGFR) mutations who had received a third-generation EGFR tyrosine kinase inhibitor.14
Additionally, in SCLC, promising progress has been reported with ADCs targeting proteins such as B7 homolog 3 protein (B7-H3), SEZ6, delta-like protein 3 (DLL3), and TROP2. SEZ6 is upregulated in approximately two-thirds of patients with primary SCLC and in those who develop resistance to platinum-based therapy after extended exposure. These characteristics make SEZ6 an attractive target for a range of patients.8 An interesting takeaway from these studies in lung cancer is that some biomarkers may coexist (ie, they are not mutually exclusive), opening the door for the development of combination approaches, depending on tolerability profiles.
Bispecific antibodies
Bispecific antibodies (BsAbs) are a rational extension of ADCs, offering the advantage of engaging two different targets or pathways simultaneously, which is especially beneficial in complex diseases like cancer. Several technological advancements in the design of BsAbs have also improved their therapeutic potential in oncology and other fields.15
BsAbs can be classified into two main categories: antigen x antigen type (ie, two antigenic epitopes) and antigen x cell-engager type (ie, one antigenic epitope and one cell-engager motif).16 The former approach can simultaneously target multiple pathways or utilise multiple biomarkers for targeted drug delivery, such as BsAbs that target both EGFR and c-MET.15 The latter is aimed at recruiting immune-cells or enhancing their function at the tumour site by targeting a specific tumour antigen and also engaging T-cell and/or natural killer (NK)-cell populations. Examples of this include bi-specific T cell engagers (BiTEs), which target CD3 on T cells and antigens such as DLL3 or the human carcinoembryonic antigen (CEA) on tumour cells. However, research and clinical trials of BiTEs in solid tumours, including NSCLC, are still in the early phases.15
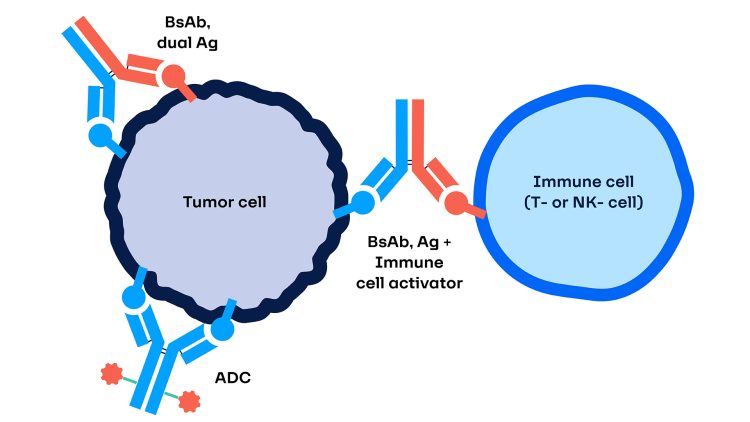

Figure 1: A schematic illustrating a bispecific antibody-drug conjugate (ADC).
A notable development in this area is the recent approval by the US Food and Drug Administration (FDA) for a BiTE targeting DLL3, aimed at treating adults with extensive-stage SCLC who have experienced disease progression on or after platinum-based chemotherapy.17
An interesting extension of BsAbs is trispecific antibodies (TsAbs), which, as the name suggests, engage three different targets – such as two immune-modulating components and a target antigen.18 Indeed, early-phase clinical studies exploring TsAbs in solid tumours have recently commenced.
Expanding treatment with combination immunotherapy
While ADCs and BsAbs can be highly personalised, combination strategies have the potential to reach a broader patient population and remain a pivotal approach for amplifying or prolonging the efficacy of lung cancer therapies, or overcoming resistance to treatment. Typically, these combinations include an immune checkpoint inhibitor (CPI; eg, anti-programmed cell death protein 1 [PD-1] / programmed cell death ligand 1 [PD-L1]) combined with other components such as chemotherapy, a targeted small molecule drug, novel CPIs, or ADCs.19,20 While novel CPI and ADC combinations are still relatively new, a well-studied example of CPI combinations is PD-1/PD-L1 with cytotoxic t-lymphocyte associated protein 4 (CTLA-4).20 More recently, early-phase studies have begun to explore combinations of standard PD-1/PD-L1 inhibitors with novel immune checkpoint inhibitors that relieve T-cell suppression (eg, anti-glycoprotein-A repetitions [GARP], anti-lymphocyte-activation gene 3 [LAG-3], and anti-T cell immunoglobulin and mucin domain-containing protein 3 [TIM3]).21,22 These combinations hold the potential to enhance and extend the durability of therapeutic outcomes for lung cancer patients.
Future perspectives
The future of lung cancer treatment is increasingly being shaped by a dual approach that seeks to harness both precision targeting and enhanced immune function, using multifunctional molecules or combinations. This strategy has the potential to offer near-term bridging solutions to address the current limitations of existing therapies and provide informative insights into new avenues for managing this challenging disease. As research advances and new technologies emerge, the potential to transform lung cancer into a more manageable condition could be on the horizon, marking a new era in the fight against this disease.
References
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-63.
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288-300.
- Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, et al. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22(4):249-74.
- Galffy G, Morocz E, Korompay R, Hecz R, Bujdoso R, Puskas R, et al. Targeted therapeutic options in early and metastatic NSCLC-overview. Pathol Oncol Res. 2024;30:1611715.
- Ashrafi A, Akter Z, Modareszadeh P, Modareszadeh P, Berisha E, Alemi PS, et al. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers (Basel). 2022;14(19).
- Friedlaender A, Perol M, Banna GL, Parikh K, Addeo A. Oncogenic alterations in advanced NSCLC: a molecular super-highway. Biomarker Research. 2024;12(1):24.
- Chagas GCL, Rangel AR, El Osta B. MET alterations in advanced non-small cell lung cancer. Curr Probl Cancer. 2024;49:101075.
- Meng Y, Wang X, Yang J, Zhu M, Yu M, Li L, et al. Antibody-drug conjugates treatment of small cell lung cancer: advances in clinical research. Discov Oncol. 2024;15(1):327.
- Aoyama M, Tada M, Yokoo H, Demizu Y, Ishii-Watabe A. Fcgamma Receptor-Dependent Internalization and Off-Target Cytotoxicity of Antibody-Drug Conjugate Aggregates. Pharm Res. 2022;39(1):89-103.
- Phuna ZX, Kumar PA, Haroun E, Dutta D, Lim SH. Antibody-drug conjugates: Principles and opportunities. Life Sci. 2024;347:122676.
- Zhou W, Xu Z, Liu S, Lou X, Liu P, Xie H, et al. Landscape of clinical drug development of ADCs used for the pharmacotherapy of cancers: an overview of clinical trial registry data from 2002 to 2022. BMC Cancer. 2024;24(1):898.
- Wiedemeyer WR, Gavrilyuk J, Schammel A, Zhao X, Sarvaiya H, Pysz M, et al. ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors. Mol Cancer Ther. 2022;21(6):986-98.
- Verma S, Breadner D, Raphael J. ‘Targeting’ Improved Outcomes with Antibody-Drug Conjugates in Non-Small Cell Lung Cancer-An Updated Review. Curr Oncol. 2023;30(4):4329-50.
- Passaro A, Janne PA, Peters S. Antibody-Drug Conjugates in Lung Cancer: Recent Advances and Implementing Strategies. J Clin Oncol. 2023;41(21):3747-61.
- Khosla AA, Jatwani K, Singh R, Reddy A, Jaiyesimi I, Desai A. Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals (Basel). 2023;16(10).
- Arasanz H, Chocarro L, Fernandez-Rubio L, Blanco E, Bocanegra A, Echaide M, et al. Current Indications and Future Landscape of Bispecific Antibodies for the Treatment of Lung Cancer. Int J Mol Sci. 2023;24(12).
- Dhillon S. Tarlatamab: First Approval. Drugs. 2024;84(8):995-1003.
- Tapia-Galisteo A, Compte M, Alvarez-Vallina L, Sanz L. When three is not a crowd: trispecific antibodies for enhanced cancer immunotherapy. Theranostics. 2023;13(3):1028-41.
- Reyes A, Muddasani R, Massarelli E. Overcoming Resistance to Checkpoint Inhibitors with Combination Strategies in the Treatment of Non-Small Cell Lung Cancer. Cancers (Basel). 2024;16(16).
- Ferrari G, Del Rio B, Novello S, Passiglia F. HER2-Altered Non-Small Cell Lung Cancer: A Journey from Current Approaches to Emerging Strategies. Cancers (Basel). 2024;16(11).
- Li A, Chang Y, Song N-J, Wu X, Chung D, Riesenberg BP, et al. Selective targeting of GARP-LTGFβ axis in the tumor microenvironment augments PD-1 blockade via enhancing CD8<sup>+</sup> T cell antitumor immunity. Journal for ImmunoTherapy of Cancer. 2022;10(9):e005433.
- Desai A, Peters S. Immunotherapy-based combinations in metastatic NSCLC. Cancer Treat Rev. 2023;116:102545.
About the author


Related topics
Antibodies, Antibody Discovery, Biomarkers, Cancer research, Clinical Trials, Drug Discovery, Drug Targets, Immuno-oncology, Immunotherapy, Precision Medicine, Target Molecule
Related conditions
Lung cancer, Non-small cell lung cancer (NSCLC), Small cell lung cancer (SCLC)
Related organisations
US Food and Drug Administration (FDA)