Building better brain models for Parkinson’s disease and beyond
Posted: 3 March 2025 | Drug Target Review | No comments yet
Professor Jens Christian Schwamborn is advancing personalised medicine for Parkinson’s disease using patient-specific brain organoids, offering new hope for more effective and targeted treatments.
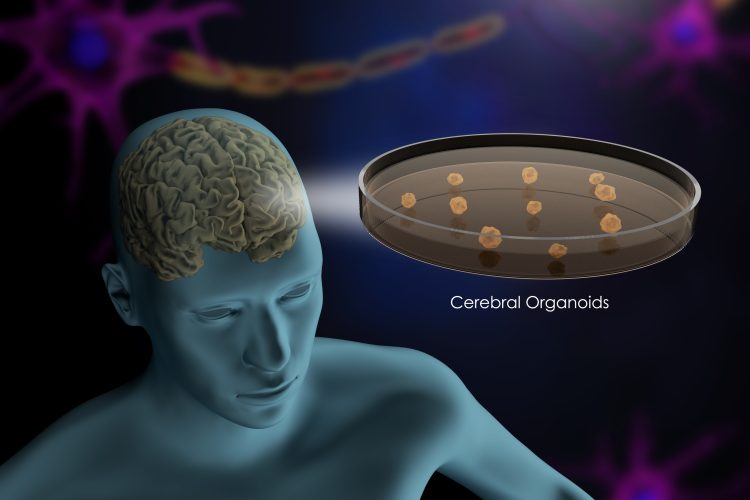

The search for effective treatments for neurodegenerative diseases like Parkinson’s disease has long been hindered by the brain’s complexity and the absence of adequate models for drug discovery. Turning this tide, Professor Jens Christian Schwamborn, a cell biology expert at the University of Luxembourg and co-founder of OrganoTherapeutics, is leading a groundbreaking effort to transform our understanding and treatment of these debilitating diseases. His research focuses on creating advanced cell culture models, such as brain organoids and assembloids, derived from patient-specific stem cells. These cutting-edge tools have the potential to accelerate drug discovery and lay the foundation for personalised medicine to treat neurological disorders.
In this interview, Professor Schwamborn discusses his research, the promise of brain organoids, and his vision for the future of stem cell-based therapies.
Building better brain models
Brain organoids are small, three-dimensional clusters of brain cells grown from stem cells in the lab. These miniaturised brain models replicate key aspects of the brain’s structure and function, enabling researchers to study brain development and neurological diseases, and to test potential treatments in a more accurate and personalised manner.
Schwamborn’s research focuses on creating patient-specific brain organoids. The process begins with a patient sample, such as blood or fibroblasts, which are reprogrammed into induced pluripotent stem cells (iPSCs). These iPSCs, derived from adult cells like blood or skin, have the unique ability to differentiate into any cell type in the body. In Schwamborn’s lab, these iPSCs are guided to develop into three-dimensional brain organoids that closely mimic the brain’s architecture and cellular composition.
The organoids are our sweet spot. This is where all our knowledge, our IP, our patents, everything is. This is where our passion lies.
“The organoids are our sweet spot,” Schwamborn explains. “This is where all our knowledge, our IP, our patents, everything is. This is where our passion lies.” His team strives to create complex models, incorporating microglia and assembling different brain regions to more accurately reflect the intricate environment of the brain. The goal is to recapitulate both healthy physiology and the pathological conditions observed in diseases like Parkinson’s disease.
From stem cell to organoid
The process of developing a usable organoid from stem cells takes time. In Schwamborn’s research, it typically takes around a month to complete the initial analysis, though it can extend to three or four months depending on the specific assay. While this timeline may seem lengthy, Schwamborn points out its wide utility, being well-suited for studying many neurodegenerative disorders. “For diseases like Parkinson’s, it’s more than sufficient,” he explains. “But for conditions like glioblastoma, it would be too slow.”
The inspiration behind the research
Schwamborn’s fascination with the brain is the driving force behind his research. “My fascination comes from the brain itself;” he explains, “simply from its complexity, from its unmet capacity.” When he began his independent research, he sought a disease model to focus on, and Parkinson’s seemed like a promising starting point. “Parkinson’s disease stood out to me because, among neurodegenerative disorders, it seemed relatively well-defined,” he says. “That’s what I thought at the time. But it turned out I was completely wrong. It’s incredibly complex.”
The advent of stem cell technology, especially the groundbreaking work of Shinya Yamanaka who won the Nobel Prize in 2012 for developing iPSCs, marked a pivotal moment. The ability to generate personalised stem cells from any individual created unprecedented opportunities for developing patient-specific disease models. “The ability to derive pluripotent stem cells in a personalised way, tailored to any individual, and use them to create cell culture models specific to one person; I found that extremely striking,” Schwamborn says.
Brain organoids: a new frontier in disease modelling
Brain organoids and assemblies represent a significant leap forward in drug discovery and disease modelling. While personalised approaches are already being used in cancer treatment, their application to neurological disorders is still in its infancy. However, Schwamborn believes that these models hold immense potential for addressing the unmet needs in this field.
We already have a significant amount of promising pilot data from ongoing pharma projects.
“There’s still a long way to go to convince pharma that this model is really useful,” he acknowledges. However, promising pilot data from ongoing collaborations with pharmaceutical companies suggest that brain organoids are set to become a valuable tool in preclinical research. “We already have a significant amount of promising pilot data from ongoing pharma projects,” Schwamborn shares. “We believe this will definitely make its way into preclinical research and practice.”
Unlocking the secrets of neurological disorders with iPSCs
Human iPSCs play a crucial role in advancing our understanding of neurological disorders, particularly Parkinson’s disease. Schwamborn highlights the importance of the personalised aspect of these models. “When we think about Parkinson’s, this is more like an umbrella term,” he explains. “Parkinson’s is diagnosed based on the symptoms, and it probably represents a huge amount of actually very different diseases that manifest with the same symptoms.”
By using personalised models, researchers can identify subgroups of patients with similar molecular characteristics, potentially leading to the development of targeted therapies. “By using personalised models, we can create patient subgroups with similar molecular characteristics, which likely means they would respond to a drug in a similar way,” Schwamborn says. This approach has the potential to revolutionise clinical trial design and lead to more effective, personalised treatments.
A vision for personalised medicine
Looking ahead, Schwamborn envisions a future where personalised medicine becomes the standard of care for neurological disorders. “In the future, when someone is diagnosed with a neurological disorder, like Parkinson’s, an organoid model would be created for that person,” he says. “Then, the organoid model would be tested with, say, five, six, or seven drugs available on the market. The patient would only receive the drug that works for their specific model, rather than the current approach where treatments are tested on the patient to see what might work.”
This approach would not only be more effective but also more cost-efficient. “We would have a much more targeted and effective approach, and it would also be economically cheaper because, from day one, the person receives the right drug,” Schwamborn explains. He believes this personalised approach has the potential to revolutionise not just the treatment of neurological disorders, but medicine as a whole.
The timeline for personalised medicine
While acknowledging that this future is not imminent, Schwamborn remains optimistic about the rapid progress in the field. “It’s definitely not going to happen tomorrow,” he says, “but I don’t think it will be beyond my retirement time. Developments are moving quickly, and we’re making significant progress. I do see this happening within a foreseeable timeframe.” He also addresses potential cost concerns, arguing that the long-term economic benefits, such as reduced workforce loss, will far outweigh the initial expenses. “When you look at the overall economics, it absolutely makes sense,” he concludes.
Professor Schwamborn’s work highlights the transformative potential of stem cell research and brain organoid technology. His vision for personalised medicine brings hope for a future where neurological disorders are treated not with a one-size-fits-all approach, but with therapies tailored to the unique needs of each patient.
Meet Professor Jens Christian Schwamborn


Related topics
Drug Discovery Processes, Induced Pluripotent Stem Cells (iPSCs), Neuroprotection, Organoids, Translational Science
Related conditions
Neurological disorders, Parkinson's disease
Related organisations
OrganoTherapeutics, University of Luxembourg
Related people
Professor Jens Christian Schwamborn