High-throughput screening capable assays in 3D
Posted: 1 May 2018 | Horst Flotow, Jane Spencer-Fry | No comments yet
High-throughput screening (HTS) has become an integral part of early stage drug discovery. This article looks at the use of the latest in vitro 3D cell and tissue culture model systems in HTS. We also highlight some of the bottlenecks and attempt a look into our crystal ball to identify some potential solutions.


TRADITIONALLY, screening assays have employed conventional 2D monolayer tissue culture in 96, 384 and even 1,536 well microtitre plates. These 2D assay systems have been optimised for throughput and convenience or cost, not necessarily biological relevance. However, in vivo cells and tissues generally grow in 3D structures that bear little resemblance to 2D monolayers. In fact, much of the physiological function of cells and tissues may rely on this 3D structure, so the game is very much afoot to develop and implement 3D cellular model systems that recapitulate the in vivo situation more closely so that it can be implemented in drug discovery. One of the key reasons for implementing 3D tissue culture in drug discovery – and HTS in particular – is to bridge the gap between the convenience of conventional 2D monolayer cell culture and the biological relevance of animal models.
According to Stratistics MRC, the Global 3D Cell Culture Market, which accounted for more than $450 million in 2016, is expected to grow to more than $1,850 million by 2023.1 This expected increase translates to an annual growth rate of more than 20%. Indeed, we have seen a rapid development in 3D culture, brought about by an increased awareness of the potential benefits this approach may bring to cell-based studies. In parallel, the number of publications citing development or use of a 3D model in cell-based research has mirrored this predicted growth. 3D models being developed have found application in target identification, drug screening, personalised medicine and regenerative medicine.
3D technology development has taken many forms including but not limited to: hydrogels, microplates, scaffolds, kits and microfluidic devices (organ-on-a-chip). Within each category there are several key manufacturers, products and devices that have been brought to market with varying degrees of success. The advantages and disadvantages of each technology lies primarily in the end result required by the researcher.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
The current state of 3D technologies
Broadly speaking, 3D technology can be divided into four categories: Hydrogels, 3D assay kits, scaffolds / microplates, and microplate technology.
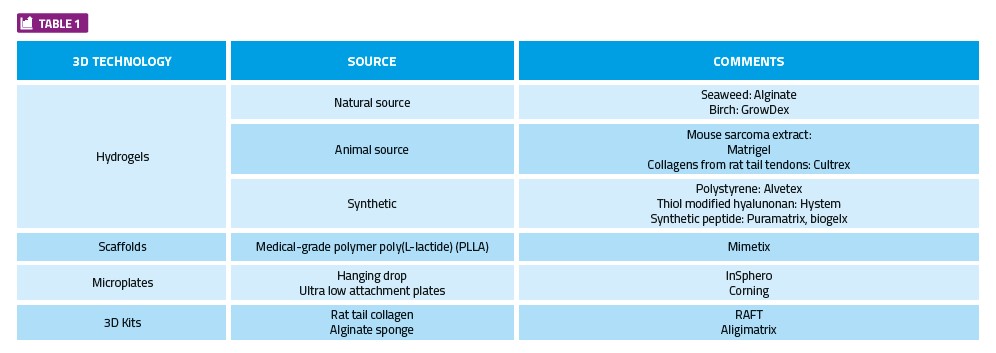
With each of the 3D technologies outlined above, some technical challenges need to be overcome before they can be broadly implemented and used for screening large compound libraries. Some current options are summarised in Table 1; however, it is by no means an exhaustive list.

3D implementation bottlenecks
This can be illustrated with perhaps the best characterised 3D technology, which is multicellular tumour spheroids. These kinds of 3D models have been used in cancer research for more than 40 years, but even here practical hurdles still exist. Spheroids can be cultured, with or without scaffolds, and in general, progress in culturing spheroids in 96- or even 384-well microtitre plates has made this a practicable, albeit low- to medium-throughput, assay method. The main bottleneck arises in the automated image acquisition and analysis.2
More recently, alternative technology and methodologies, such as different forms of hydrogel, have come to the market. The use of hydrogels for this type of assay has enabled higher sample throughput, in addition to reducing the number and complexity of steps required. With suitable hydrogels, it is now possible to carry out simple 3D tissue culture in 384-well microtitre plates and ramp up the throughput to medium to high. The automated image acquisition and analysis still requires some optimisation, but this appears to represent the most likely way forward.
Hydrogels to the fore
Data to be acquired during the study (eg, cell-cell interaction, migration, organoid formation) and the end goal (eg, end point assay, cell recovery, downstream processing) will dictate the technology best suited for the job. In HTS applications where imaging technology is employed for data acquisition, hydrogels have an advantage. This is also true for studies where samples need to be recovered for further downstream processing, such as RNA and DNA analysis.
In general, the key characteristics for consideration when selecting the appropriate 3D technology, as well as some examples of available products, are provided in Table 2.
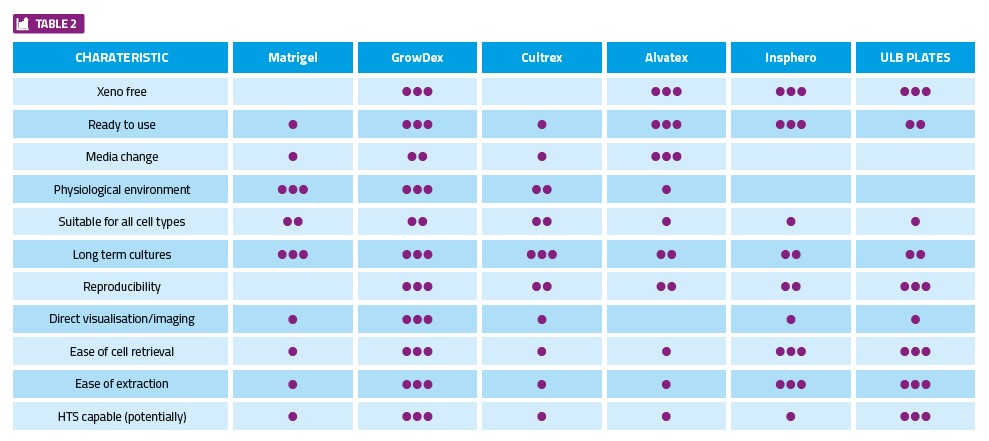

Clearly there will always be a need to make HTS assays better and more biologically relevant. Recent developments in 3D tissue culture models provide cause for cautious optimism, but the cellular model chosen must be fit for purpose. The following few basic considerations may help when selecting the most appropriate technology and smooth the path to successful implementation:
- Simplicity: This sounds obvious, yet it deserves the most attention. Is the model chosen not only fit for purpose, but one that most people in the lab can set up and run? Organ-on-a-chip may sound good, but if it is so complicated that one cannot easily transfer the technology or demonstrate its utility to a broader community its use may be limited.
- Robustness and reproducibility: Is the methodology simple to replicate between batches and operators? If not, it will be difficult to compare to other models and to standardise across different laboratories. Robustness and reproducibility are key to establishing such measurements on a routine basis, particularly for toxicity measurements.
- Infrastructure requirements: 3D tissue culture requires considerable investment in hardware and IT infrastructure to run at HTS level. Make informed choices to ensure instrumentation is future proof and data storage, handling and processing has been thoroughly considered.
Other kinds of 3D model systems that are currently more of a vision/wish list for future drug discovery using HTS, include the following:
- Organoid culture models: As the name implies, organoids are basically 3D in vitro cellular micro-tissue model systems that display characteristics of organ.3 Organoids are the most technically challenging 3D in vitro models and, until recently, have not been utilised in any HTS studies. Due to the many technical hurdles, this highly specialised endeavour has only been implemented in a small number of laboratories. Therefore, making comparisons on assay robustness and reproducibility (day-to-day and lab-to-lab) is currently somewhat challenging.
- Whole animal HTS: This is currently only really practical with one of the smallest and best characterised animals, the nematode Caenorhabditis elegans.4 This approach seems to be gaining momentum, as the model system C. elegans has been well characterised genetically and, thus, can find applications in drug discovery ranging from simple toxicity measurements, oncology and ageing to anti-infectives.5
Summary and conclusions
Drug discovery requires the use of high throughput capable, simple and robust assays that have high biological relevance. The 3D tissue culture techniques being developed are now starting to meet these HTS criteria with varying degrees of success.
Hydrogels and scaffold technologies have enabled relatively straightforward 3D cell-based assays to be set up in microtitre plates for screening purposes.
The main bottleneck appears to have now moved to the acquisition (automated image acquisition, for example) and analysis of the data from such 3D cultures. This bottleneck can inevitably be resolved but may require considerable investment in time and resources.
Organoid cultures, although not yet routinely used in HTS, could move into the mainstream in the next few years given the pace of technological progress in this field. Whole animal HTS using C. elegans is feasible and could be more widely deployed in the near future in some therapeutic indications.
References
1. Orbis 3D report (2017). 3D Cell Culture-Global Market Outlook (2017-2023). http://orbisresearch.com/reports/index/3d-cell-culture-global-market-outlook-2017-2023.
2. Kriston-Vizi J, Flotow H. (2017). Getting the whole picture: High content screening using three-dimensional cellular model systems and whole animal assays. Cytometry. Part a: the Journal of the International Society for Analytical Cytology, 91(2), 152–159.
3. Barker N. (2014). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature Reviews Molecular Cell Biology, 15(1), 19–33. http://doi.org/10.1038/nrm3721.
4. Kobet RA, Pan X, Zhang B, Pak SC, Asch AS, Lee MH. (2014). Caenorhabditis elegans: A Model System for Anti-Cancer Drug Discovery and Therapeutic Target Identification. Biomolecules and Therapeutics, 22(5), 371–383. http://doi.org/10.4062/biomolther.2014.084
5. Lakshmanan U, Yap A, Fulwood J, Yichun L, Hoon S, Lim J, et al. (2014). Establishment of a Novel Whole Animal HTS Technology Platform for Melioidosis Drug Discovery. Combinatorial Chemistry & High Throughput Screening, 17(9), 790–803. http://doi.org/10.2174/1386207317666141019195031
Biography
JANE SPENCER-FREY has a degree in Biochemistry and PhD in molecular biology with more than 30 years experience in the Life Science sector. She has been involved in both large international corporations and startups where the focus has been on bringing new innovative technology, instrumentation, reagents and software to the market. Previous positions have included Director of European Operations for BioVeris, and CEO for Chip-Man Technologies. Currently, Jane holds the position of Head of Commercialisation at UPM Biomedicals, where she is responsible for developing company initiatives in cell culture and wound care areas.


The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related topics
Assays, High-Throughput Screening (HTS)
Related people
Horst Flotow, Jane Spencer-Fry