Antibody library screening: high throughput strategies
Posted: 10 June 2020 | Emma Cummins - Centre for Drug Research and Development Canada, Gregorio Aversa - Abimmune Technologies Inc., Ismael Samudio - Centre for Drug Research and Development Canada | No comments yet
As the number of antibody discovery platforms and formats expands, various characteristics must be rigorously screened for and taken into consideration when deciding which hits to progress to successful development. The earlier in the antibody discovery pathway the assessment is carried out, the greater the hit enrichment and selection will be, leading to more controlled attrition along the pipeline. In this review we will discuss phage display antibody library screening challenges, and commonly used high-throughput screening methods that can be applied to solve them.

HTS for Antibody-Drug Conjugates (ADC)
ADC are antibodies that have been synthetically functionalised with a cytotoxic payload (typically microtubule inhibitors) covalently attached to a chemical linker. Currently there are three FDA-approved ADC: brentuximab vedotin (Adcetris®, Seattle Genetics) targeting CD30 for treating Hodgkin’s lymphoma; trastuzumab emtansine (Kadcyla®, Genentech/Roche) targeting HER2 for treating HER2-positive metastatic breast cancer; and gentuzumab ozogamicin (Mylotarg®, Pfizer), targeting CD33 for treating acute myelogenous leukemia (AML). Mechanistically, ADC have a requirement for their target antigen to be internalised upon binding so that the cytotoxic payload of target:ADC can be delivered intracellularly – typically to the lysosome/ endolysosome where the linker can be efficiently cleaved by resident proteases.16,17
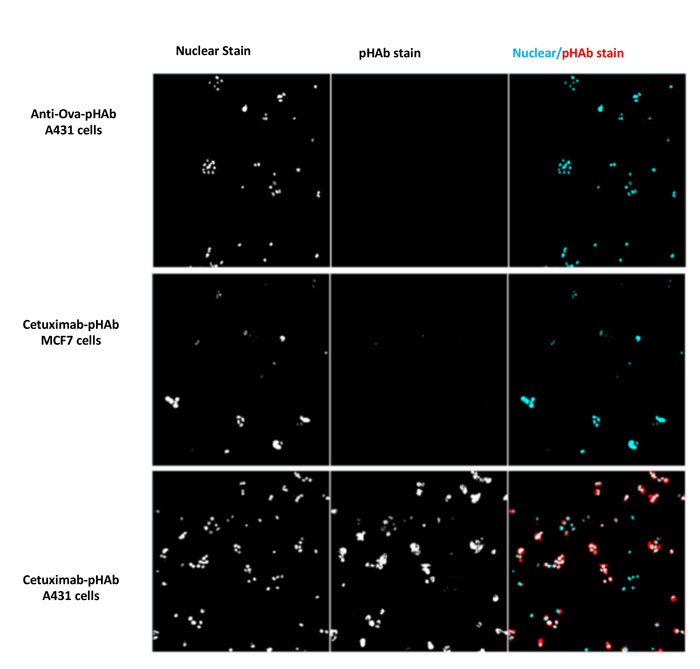

Figure 3: A431 cells (EGFR positive) and MCF7 cells (EGFR negative) were treated with pHAb-conjugated Cetuximab (anti-EGFR IgG) or an antiovalbumin IgG, and incubated for 24 h before imaging on a HCS platform. The pHAb dye fluoresces due to the pH change when the pHAblabelled Cetuximab is internalised into the cell.
In our experience, not all antibodies targeting surface molecules internalise equally, if at all. Thus, guiding the screening process earlier towards effectively internalised antibodies can save considerable time and resources downstream. The gold standard measure of therapeutic antibody internalisation is localisation of the Fc region of a fluorescently labelled antibody by confocal microscopy; however, performing confocal microscopy evaluation for dozens or hundreds of candidates is very costly and time-consuming. Alternatively, a slightly higher throughput readout is cellular cytotoxicity of ADCs which can be monitored in microtiter plates. However, generating ADC for dozens of antibodies is impractical, and using secondary antibodies bearing cytotoxic payloads is costly and does not reflect the physiology or kinetics of single antibody / payload. Instead, recent advances in the development of pH-sensitive dyes and ‘click’ chemistry have enabled high-throughput assessment of antibody internalisation by monitoring the fluorescence changes of antibody-fluorochrome conjugates. In this approach, microgram quantities of dozens or hundreds of antibodies can be chemically coupled – via lysines or free thiols using on-bead conjugation strategies – to a pH sensitive fluorochrome such as pHAb (Promega) or pHrodo (Thermo Fisher) with minimal to no purification. Antibody-fluorochrome conjugates are then incubated with target cells on microtiter plates, along with adequate controls (target-negative cells, labeled isotype controls, etc.) for 1 – 24 h in culture, and pH-dependent increases in fluorescence, indicative of lysosomal / endolysosomal trafficking, are read by flow cytometry, plate fluorescence or high content plate imaging (Figure 3). We found this approach provides the most cost-effective and high-throughput means to identify internalising antibodies that, together with other developability criteria, can effectively select ADC therapeutic candidates. Recently, a parallel-conjugation method for antibodies in 100 μg scale in 96-well format has been described for maytansinoid class of ADC, requiring <5ug of ADC for characterisation.18 This method may greatly increase the ADC screening throughput in cellular assays and could be employed in early-stage ADC screening.
HTS for ADCC enabled antibodies
Fc-mediated recognition of opsonized cellular targets by effector immune cells – typically NK cells – can result in antibody-dependent, cell mediated cytotoxicity (ADCC). ADCC is mediated by cross-linking of the FcIIIA receptor (CD16a) on NK cells by IgG1 Fc domain of antibodies bound to the target cells, which triggers degranulation of NK cells and release of perforin and cytolytic proteases that induce lysis of target cells. Several FDA-approved monoclonal antibodies have been shown to exert their therapeutic action, in part via ADCC: rituximab – an anti-CD20 antibody for treating non-Hodgkin’s lymphoma; obinutuzumab – an anti-CD20 antibody for treating chronic lymphocytic leukemia; dinituximab – an anti-GD2 antibody for treating high-risk neuroblastoma; trastuzumab – an anti-HER2 antibody for treating breast and metastatic gastric cancer; and cetuximab – an anti-EGFR antibody for treating metastatic colorectal and head and neck cancers.19
The overarching requirement for adequate ADCC is the formation of a proper immunological synapse leading to crosslinking of CD16, and downstream activation of the transcription factor NFAT. A commonly employed high-throughput approach utilises Jurkat cells stably transduced with CD16a and an NFAT-responsive luciferase (or GFP/RFP) incubated in microtiter plates with target cells previously opzonised with test IgGs. Reporter gene expression is read 12-48 h later in a high-throughput platform, typically a luminescent plate reader, after adding substrate, or a fluorescent plate reader if the reporter is GFP or RFP. This method is extremely robust, cost-effective and reproducible, since it uses a homogeneous cell line instead of primary effector cells which can give variable results. However, the Jurkat reporters may not always be the best choice, particularly when testing in the context of other stimuli such as checkpoint modulators, immune adjuvants, etc. that differentially affect cancer cell lines (like Jurkats) or require other signaling pathways to be operational in the effector cells (like PD-1, Tim 3, etc.). Applying high-throughput screening strategies to assess function and developability in large panels of diverse hits – as early as possible in the antibody campaign – can inform hit selection, and enable early attrition where required, while preserving sufficient diversity and desired target product profiles. Advances in technology have facilitated the examination of these properties, and will hopefully accelerate the pathway to successful candidates.
References
- Rossant CJ, Matthews C, Neal F, Colley C, Gardener MJ, Vaughan T. Versatility of homogeneous time-resolved fluorescence resonance energy transfer assays for biologics drug discovery. J Biomol Screen. 2015;20(4):508-18.
- Finlay WJ, Cunningham O, Lambert MA, Darmanin-Sheehan A, Liu X, Fennell BJ, et al. Affinity maturation of a humanized rat antibody for anti-RAGE therapy: comprehensive mutagenesis reveals a high level of mutational plasticity both inside and outside the complementarity-determining regions. J Mol Biol. 2009;388(3):541-58.
- Cummins E, Luxenberg DP, McAleese F, Widom A, Fennell BJ, Darmanin-Sheehan A, et al. A simple high-throughput purification method for hit identification in protein screening. J Immunol Methods. 2008;339(1):38-46.
- Xiao X, Chen Y, Mugabe S, Gao C, Tkaczyk C, Mazor Y, et al. A Novel Dual Expression Platform for High Throughput Functional Screening of Phage Libraries in Product like Format. PLoS One. 2015;10(10):e0140691.
- Brooks BD, Miles AR, Abdiche YN. High-throughput epitope binning of therapeutic monoclonal antibodies: why you need to bin the fridge. Drug Discov Today. 2014;19(8):1040-4.
- Sivasubramanian A, Estep P, Lynaugh H, Yu Y, Miles A, Eckman J, et al. Broad epitope coverage of a human in vitro antibody library. MAbs. 2017;9(1):29-42.
- Friis LM, D. Developability assessment of therapeutic antibodies. Drug Target Review. 2017.
- Miller BR, Demarest SJ, Lugovskoy A, Huang F, Wu X, Snyder WB, et al. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel. 2010;23(7):549-57.
- Fennell BJ, McDonnell B, Tam AS, Chang L, Steven J, Broadbent ID, et al. CDR-restricted engineering of native human scFvs creates highly stable and soluble bifunctional antibodies for subcutaneous delivery. MAbs. 2013;5(6):882-95.
- Avery LB, Wade J, Wang M, Tam A, King A, Piche-Nicholas N, et al. Establishing in vitro in vivo correlations to screen monoclonal antibodies for physicochemical properties related to favorable human pharmacokinetics. MAbs. 2017:1-12.
- Frese K, Eisenmann M, Ostendorp R, Brocks B, Pabst S. An automated immunoassay for early specificity profiling of antibodies. MAbs. 2013;5(2):279-87.
- Kelly RL, Sun T, Jain T, Caffry I, Yu Y, Cao Y, et al. High throughput cross-interaction measures for human IgG1 antibodies correlate with clearance rates in mice. MAbs. 2015;7(4):770-7.
- Liu Y, Caffry I, Wu J, Geng SB, Jain T, Sun T, et al. High-throughput screening for developability during early-stage antibody discovery using self-interaction nanoparticle spectroscopy. MAbs. 2014;6(2):483-92.
- Buck PM, Kumar S, Wang X, Agrawal NJ, Trout BL, Singh SK. Computational methods to predict therapeutic protein aggregation. Methods Mol Biol. 2012;899:425-51.
- Jain T, Sun T, Durand S, Hall A, Houston NR, Nett JH, et al. Biophysical properties of the clinical-stage antibody landscape. Proceedings of the National Academy of Sciences. 2017;114(5):944-9.
- Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35(4).
- Caculitan NG, Dela Cruz Chuh J, Ma Y, Zhang D, Kozak KR, Liu Y, et al. Cathepsin B Is Dispensable for Cellular Processing of Cathepsin B-Cleavable Antibody-Drug Conjugates. Cancer Res. 2017;77(24):7027-37.
-
Catcott KC, McShea MA, Bialucha CU, Miller KL, Hicks SW, Saxena P, et al. Microscale screening of antibody libraries as maytansinoid antibody-drug conjugates. MAbs. 2016;8(3):513-23.
-
Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front Immunol. 2015;6:368.
Biographies




ISMAEL SAMUDIO obtained his PhD in genetics from Texas A&M University in 2002, and received postdoctoral training in Immunotherapy at the BC Cancer Agency. Ismael joined CDRD in 2015 and participated in the development of various immunotherapy platforms, including a novel targeting arm for CAR-T cells with potential for therapeutic efficacy against various solid tumors. Ismael is currently the Head of the Biologics Division at CDRD and has published over 70 peer reviewed articles and various book chapters on the use of small molecules, cellular therapeutics, and immunotherapy strategies for the treatment of cancer.


The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related organisations
Abimmune Technologies Inc., Centre for Drug Research and Development Canada