Urine-derived stem cells as innovative platform for drug testing and disease modelling
Posted: 20 July 2018 | Audrey Ncube, James Adjaye (Heinrich-Heine-University), Lisa Nguyen, Lucas-Sebastian Spitzhorn, Martina Bohndorf, Shaifur Rahman, Wasco Wruck (Heinrich-Heine-University) | No comments yet
Kidneys are crucial for filtration of drugs and toxins and their proper function is essential for overall health. Unfortunately, due to disease and improper function, kidney transplantation or dialysis are necessary for millions of patients annually all over the world.1,2
Several drugs are nephrotoxic per se. These side effects could be increased by prolonged medication and synergistic effects with other drugs, such as cephalosporins plus aminoglycosides, which are used in cancer treatment.11 Although there are models such as cisplatin treatment12 to investigate drug-induced nephrotoxicity, more prospective studies and novel in vitro models are warranted to validate these approaches.
Embryonic and induced pluripotent stem cells (ESCs / iPSCs) have been differentiated into kidney-related cells, thus representing a potential alternative to native cells.13-15
The kidney is an organ of high complexity consisting of many cell types.16 One key regulator of nephrogenesis, SIX2, has been shown to be expressed in umbilical cord mesenchymal stem cells (uMSCs).17 Bone marrow-derived mesenchymal stem cells (MSCs) have been successfully used to restore renal function deficits.18 It has been shown that renal cells can be isolated from urine as well as from third-trimester amniotic fluid, which mostly consists of foetal urine. Obtaining kidney biopsy-derived cells from patients is difficult; thus urine represents a substitution which enables personalised research approaches for kidney-related drug screening and toxicity tests. Building upon previous work showing that amniotic fluid mostly consists of foetal urine,19 amniotic fluid-derived stem cells were recently also described as MSCs of renal origin20 and have been used to generate podocytes.21
Urine is a source of stem cells
Derivation of patient-specific stem cells from urine has many advantages over other established stem cell sources. These autologous or human leukocyte antigens (HLA)-matched uMSCs are ideal for transplantation purposes. Additionally, it is possible to generate vast amounts of these cells because the source is nearly infinite and can be expanded in vitro on a large scale in a good manufacturing practice (GMP) setting. In the case of infants and older patients with impaired wound healing, urine cells can be obtained non-invasively, without wounding, cost-effectively and easily cultured. uMSCs or uRETCs can be reprogrammed into iPSCs17 also under GMP conditions.
Urine-derived stem cells have features of MSCs and kidney cells
Cellular and molecular characterisation of the uMSCs and uRETCs revealed similarities with amniotic fluid-derived MSCs.20 Furthermore, they are able to differentiate into osteoblasts, adipocytes and chondrocytes and secreted cytokines capable of modulating the immune system and support healing processes. Besides their MSC marker expression, these cells express kidney-related markers such as SIX2, Nephrin and LHX1 and can be differentiated into podocyte-like cells (Figure 1). Furthermore, it was possible to efficiently generate iPSCs from these cells.17 These uMSC-iPSCs can be differentiated into all cell types within the body including hepatocytes, neurons and endothelial cells (Figure 1).
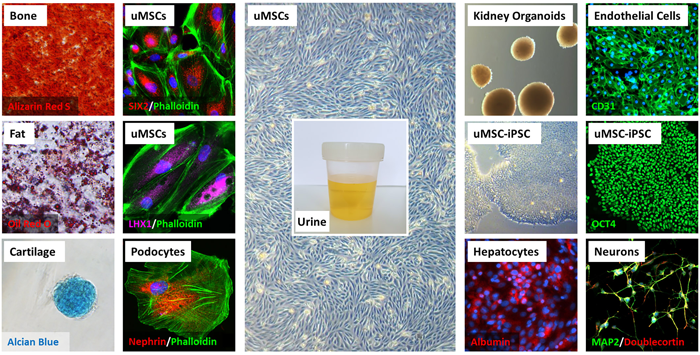

FIGURE 1: Molecular and cellular characteristics of uMSCs and uMSC-iPSCs. (Middle column) uMSCs with grain-like morphology were isolated from urine. (Left columns) They are able to differentiate into fat (Oil Red O), cartilage (Alcian Blue) and bone cells (Alizarin Red S) and additionally express renal markers SIX2, LHX1 and Nephrin and can be differentiated into podocyte-like cells. (Right columns) uMSC-iPSCs are positive for OCT4 and can be differentiated into endothelial cells (CD31), neuronal cells (MAP2 and Doublecortin), B IOGRAPHY hepatocytes (Albumin) and form kidney organoids. Cell nuclei were stained using DAPI or Hoechst 33258
Gene expression analysis confirms kidney origin of urine cells
Figure 2 shows a heatmap representation of genes associated with (A) pluri- and multipotency, (B) renal developmental processes, (C) encoding the core ADME (absorption, distribution, metabolism, and excretion) enzymes and (D) of the cytochrome P450 family. The heatmaps are based on uMSC transcriptome data sets described in the research17,20 and also uRETCs obtained directly from urine using a selective medium for differentiated cells. Drugs up-regulate the enzyme systems responsible for their degradation or activation (Phase I) and conjugation (Phase II) to biochemical forms that can be excreted in bile or urine.22-24 Genes coding Phase I and Phase II enzymes are included in the absorption, distribution, metabolism, and excretion (ADME) genes. The high similarity between both cell types is demonstrated by the predominantly consistent expression between both cell types. However, clustering of renal developmental genes shows a separation of uMSCs and uRETCs pointing to differences in these cells in nephrogenesis. These differences are shown in Figure 2E. The subsets of genes exclusively expressed in uMSCs or uRETCs were further subjected to gene ontology (GO) analysis and revealed differences in kidney developmental processes, such as the GO-terms drug transmembrane transport, paramesonephric duct development, distal tubule morphogenesis for uRETCs and metanephric tubule development, glomerulus development for uMSCs (Figure 2F).
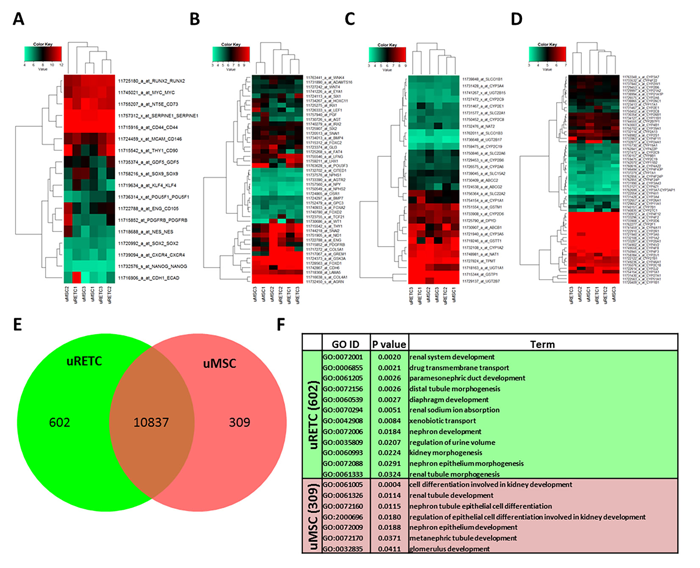

FIGURE 2: Gene expression profiles characterise renal developmental properties of uMSCs and uRETCs. Heatmap-based representations of co-regulated expression of distinct gene sets associated with (A) pluri- and multipotency, (B) renal developmental processes, (C) the core ADME genes (http://pharmaadme.org/joomla/index.php?option=com_content&task=view&id=14&Itemid=29) and (D) cytochrome P450 genes. uMSCs and uRETCs have high similarity. However, the clustering related to renal development demonstrates differences between both cell types (C). Values on the color key scales are logarithmic (base 2). (E) Venn diagram analysis of genes expressed in uMSCs and uRETCs identified 10837 genes expressed in common, 309 genes expressed in uMSCs and 602 genes expressed in uRETCs. (F) Genes exclusively expressed in uMSCs or uRETCs reveal many kidney-related terms which point to their distinct developmental potential.
Improve disease modelling, drug screening and nephrotoxicity tests using 3D-based kidney organoids
Ethical concerns and the limited predictability of cross-species translation of research results increase the importance of in vitro models as an alternative to in vivo pre-clinical testing. Recent 3D approaches increase assay relevance but still need improvement.25
Moreover, uMSC-iPSCs are the basis for generating 3D kidney organoids. To resemble the complex kidney structure, it is useful to combine different cell types. We have generated 3D kidney organoids (Figure 1) by combining uMSCs as renal cells with uMSC-iPSCs differentiated endothelial cells (iECs) and mesenchymal stem cells (iMSCs) originating from the same individual.17 This approach may enable researchers to better mimic human physiology in contrast to standard 2D cell culture systems. For future research and therapy, it is important to increase maturity, and thus functionality, in order to attain predictive value. Recent studies have shown bio-printing26,27 and kidney-on-a-chip technology28 as improved means of generating better in vitro models.
Perspectives
Urine is a source of stem cells that can be accessed non-invasively, with no pain and risk for the patient; has nearly unlimited availablity; and is cost-efficient. Urine should be considered as an alternative to established stem cell sources such as bone marrow and cord blood. Most importantly, uMSCs and uRETCs originate from the kidney and, thus, by-pass the need for donated kidneys. Combined with HLA analysis and analysis of the patient‘s CYP variants, the cells are perfectly suited for individualised drug screening, dose determination, disease modelling in vitro and eventually kidney-associated cell therapies.
Acknowledgements
James Adjaye acknowledges support from the medical faculty of the Heinrich Heine University- Düsseldorf, Germany. Md Shaifur Rahman acknowledges support from the German Academic Exchange Service (DAAD-91607303).
References
- Kellum JA, Hoste EA. Acute kidney injury: epidemiology and assessment. Scand J Clin Lab Invest Suppl 2008;241:6–11.
- Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 2008;36(4 Suppl):S146–S151.
- Saidi RF, Kenari SKH. Challenges of Organ Shortage for Transplantation: Solutions and Opportunities. Int J Organ Transplant Med. 2014;5(3):87–96.
- Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4:1275–1283.
- Koyner JL, Cerdá J, Goldstein SL, Jaber BL, Liu KD, Shea JA, et al. Acute Kidney Injury Advisory Group of the American Society of Nephrology. The daily burden of acute kidney injury: a survey of U.S. nephrologists on World Kidney Day. Am J Kidney Dis. 2014 Sep;64(3):394-401.
- Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, Chakaravarthi R, Holden AL, Goldstein SL. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015 Aug;88(2):226-34.
- Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66.
- Izzedine H, Perazella MA. Onco-nephrology: an appraisal of the cancer and chronic kidney disease links. Nephrol Dial Transplant. 2015;30: 1979–1988.
- Awdishu L, Mehta RL. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017 Apr 3;18(1):124.
- Sanchez-Romero N, Schophuizen CM, Gimenez I, Masereeuw R. In vitro systems to study nephropharmacology: 2D versus 3D models. Eur J Pharmacol 2016;790:36–45.
- Izzedine H, Perazella MA. Anticancer Drug-Induced Acute Kidney Injury. Kidney Int Rep. 2017 Feb 16;2(4):504-514.
- Bulacio RP, Torres AM. Organic anion transporter 5 (Oat5) renal expression and urinary excretion in rats treated with cisplatin: a potential biomarker of cisplatin-induced nephrotoxicity. Arch Toxicol. 2013;87(11):1953–62.
- Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013 Dec;15(12):1507-15.
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53-67.
- Little MH. Generating kidney tissue from pluripotent stem cells. Cell Death Discovery. 2016;2,16053.
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61(2):387-95.
- Bohndorf M, Ncube A, Spitzhorn LS, Enczmann J, Wruck W, Adjaye J. Derivation and characterization of integration-free iPSC line ISRM-UM51 derived from SIX2-positive renal cells isolated from urine of an African male expressing the CYP2D6 *4/*17 variant which confers intermediate drug metabolizing activity. Stem Cell Res. 2017 Dec;25:18-21
- Hamza AH, Al-Bishri WM, Damiati LA, Ahmed HH. Mesenchymal stem cells: a future experimental exploration for recession of diabetic nephropathy. Ren Fail. 2017;39(1):67-76.
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–348.
- Rahman MS, Spitzhorn LS, Wruck W, Hagenbeck C, Balan P, Graffmann N et al. The presence of human mesenchymal stem cells of renal origin in amniotic fluid increases with gestational time. Stem Cell Res Ther. 2018 Apr 25;9(1):113.
- Xinaris C, Benedetti V, Novelli R, Abbate M, Rizzo P, Conti S, et al. Functional human podocytes generated in organoids from amniotic fluid stem cells. J Am Soc Nephrol. 2016;27(5):1400-11.
- Graffmann N, Matz P, Wruck W, Adjaye J. The promise of induced pluripotent stem cells for liver disease, toxicology and drug discovery. Drug Target Rev. 2, 8–11 (2015).
- Jozefczuk J, Prigione A, Chavez L, Adjaye J. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev. 2011 Jul;20(7):1259-75.
- Matz P, Wruck W, Fauler B, Herebian D, Mielke T, Adjaye J. Footprint-free human fetal foreskin derived iPSCs: A tool for modeling hepatogenesis associated gene regulatory networks. Sci Rep. 2017 Jul 24;7(1):6294.
- Nguyen DG, Pentoney SL Jr. Bioprinted three dimensional human tissues for toxicology and disease modeling. Drug Discov Today Technol. 2017 Mar;23:37-44.
- King SM, Higgins JW, Nino CR, Smith TR, Paffenroth EH, Fairbairn CE et al. 3D Proximal Tubule Tissues Recapitulate Key Aspects of Renal Physiology to Enable Nephrotoxicity Testing. Front Physiol. 2017 Mar 8;8:123
- Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 2016;6:34845.
- Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R. Kidney-on-a-Chip Technology for Drug-Induced Nephrotoxicity Screening. Trends Biotechnol. 2016 Feb;34(2):156-170.
Biography














The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related topics
Disease Research, Genetic Analysis, Stem Cells
Related conditions
Kidney disease
Related organisations
Heinrich Heine University Düsseldorf







