Antibody fragment technology and avian IgY antibodies: a powerful combination
Posted: 5 February 2016 | Carol Harley (IBMC and i3S), Ricardo Vieira-Pires (University of Coimbra) | No comments yet
Recombinant monoclonal antibodies or antibody biologics have been successfully developed as diagnostic and therapeutic agents…


These antibodies are generally mammalian in origin (Figure 1A) and have been selected from naïve or immune libraries for their high binding affinity and their ability to modulate the activity of their biological target. In parallel, within the research laboratory, polyclonal and monoclonal antibodies are routinely used in western blotting techniques, ELISA assays, immunoprecipitations and localisation of proteins within cells, usually by fluorescence conjugation. Classically, antibodies are raised in rabbits, horse, donkey and mice; however, despite their widespread use suffer from a series of disadvantages such as variable cost, issues of cross-reactivity, inconsistency between batches and limited supply of the isolated antibody. In this review we will highlight the powerful combination of using phage display to produce customised recombinant antibody fragments for research and the advantages of exploring the avian immune repertoire for the generation of novel research tools.
Recombinant antibody libraries: case studies
Antibody fragments were originally defined as stable proteolytic cleavage products of full-length antibodies. Some of these fragments – Fab, Fv (Figure 1B) – retain the ability to recognise an epitope with great specificity and affinity while becoming less complex at the molecular level, displaying less flexibility and simpler folding. These properties make antibody fragments the ideal molecules for use as crystallisation chaperones in structure determination of proteins, with particular success in the case of membrane proteins2-5.
In addition, the properties of antibody fragments make them amenable for the generation of recombinant libraries that can be explored using careful selection strategies. An example of the application of this approach includes the selection of single chain variable fragment (scFv) antibodies that recognise specific conformational states of GPCR proteins6. Another application is the selection of antibody fragments that identify key intermediates in pathways leading to amyloid formation and disease progression. Neurodegenerative diseases such as Parkinson’s disease, Huntington’s disease, Creutzfeldt-Jakob disease and Alzheimer’s disease are disorders typified by aberrant expression of an insoluble form of a normally soluble protein which leads to protein aggregation and deposits of amyloid fibrils in a variety of organs and tissues7.
Normal Huntington protein (HTT) is expressed within the cell cytoplasm whereas the mutant Huntington protein (mHTT), which has elongated poly-glutamine (polyQ) repeats, undergoes abnormal folding, proteolytic cleavage and aggregation which eventually gives rise to neuronal inclusion bodies8. It has been shown that the regions surrounding the polyQ tract also regulate the toxicity of the protein; the first 17 amino acids promote aggregation whereas the C terminal proline rich region inhibits protein aggregation. A naïve human spleen scFv phage-display library was screened against the N-terminal 17 amino acids of HTT and the scFv-C4 intrabody was selected9. This intrabody is conformational-specific as it does not recognise the wild-type HTT protein, maybe due to epitope accessibility. The scFv-C4 antibody was re-engineered to increase its folding stability in the cell cytoplasm and was shown to bind to the mHTT protein and reduce aggregation. Furthermore, addition of a C terminal proteosomal targeting sequence (PEST) to scFv-C4 has demonstrated that the mHTT protein can be successfully targeted for degradation, removing toxic forms of mHTT from cells10. The molecular details of how scFv-C4 interacts with the first 17 amino acids of HTT have recently been revealed in the co- crystallisation and NMR structures of these complexes11. Another example is focused on α-synuclein protein, a natively unfolded cytoplasmic protein which in a mutated form can exist as small aggregates, larger oligomers or Lewy bodies linked with Parkinson’s disease. It has been demonstrated that morphologically distinct conformations of a-synuclein can be distinguished by isolated scFv fragments; D5-scFv which recognises dimeric and tetrameric forms12 whereas syn10H-scFv antibody binds trimeric and hexameric aggregates13.
An important advantage of the use of molecules selected from recombinant antibody libraries is the possibility of re-engineering the selected molecules by adding new domains that confer specific properties to the antibody-target complex. A clear example was described above, where a sequence targeting the protein to the proteasome was engineered at the C-terminus of the antibody so that its cognate partner would be directed for degradation. Other possible modifications include chemical modification by reactive moieties such as fluorescent probes and the creation of antibody fragment fusions with a SNAP-tag domain for fluorescence labelling or with mutant BirA ligase, a biotinylating-enzyme, for a proximity assay. The clear message is that antibody fragment technology is a very powerful tool to be used in the study of molecular processes.
Attributes of the Avian Model for generation of antibodies
An apparent stumbling block for the widespread use of recombinant antibody fragment libraries is the perceived difficulty involved in their creation. However, advances in molecular immunology, antibody engineering and transgenic animals have opened new opportunities to explore birds, such as chickens, geese and quails, for antibody discovery campaigns. Indeed, birds have unique mechanisms of immune diversification and a distinctive organisation of their germline antibody genes enabling fast generation of robust recombinant antibody fragments.
The use of avian antibodies as research tools is not a novel concept. Historically, the presence of protective avian antibodies (IgY) in the yolk of immunised hens was demonstrated by Klemperer in the late 1800s14. This observation was largely neglected until the 90s when the need to refine alternatives to animal testing boosted the IgY-technology15. As a consequence, specific antibodies directly extracted from egg yolk have been successfully used in research (Figure 2), diagnostics and therapies16 and holds great potential for the fields of biomedicine and biotechnology17.


A clear advantage of using avian antibodies relates to the evolutionary distance from different mammalian species. The phylogenetic distance that separates humans from birds is about 300 million years (Myr) contrasting with the 90 Myr of other mammalian species18. It has been shown that the immune response in an antibody-producing animal tends to increase as its phylogenetic difference with the animal used as the antigen source increases. For this reason it is more likely to obtain a robust immune response against highly conserved mammalian proteins from an avian host.
Genetic and functional studies of the IgY molecule position it as common ancestor to mammalian IgG and IgE23. Overall, the IgY molecule keeps a common immunoglobulin architecture, with two heavy and two light chains (Figure 1A). However, IgY rather resembles mammalian IgE, having a higher molecular mass (170KDa) due to the presence of an extra constant region (CH2) in the heavy chain; it also lacks a flexible hinge-region, harbours identical CH1/CH2 interchain disulphide bond patterns and additional glycosylation sites24. At the level of the antigen recognition and binding sites, avian IgY antibodies tend to present larger complementarity determining regions (CDR loops) than mammals and present a unique population of cysteine-rich CDRs, which are particularly observed in CDR3 of the variable heavy chain25. These CDR differences are only now being thoroughly explored at the structural level26, revealing their full potential for the generation of antibodies, unlike the ones from mammalian sources.
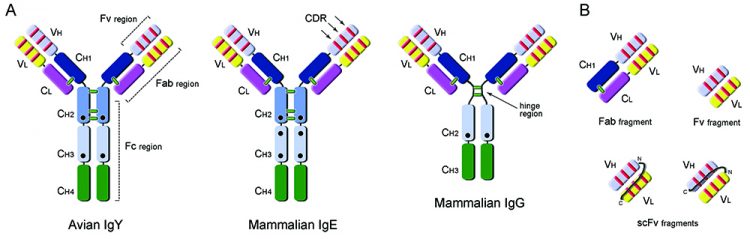

Figure 1: Antibody and recombinant fragment structures. A: Comparison of domain organisation of avian IgY (MW: ~170KDa) antibody with mammalian IgE (MW: ~190KDa) and IgG (MW: ~150KDa) antibodies. IgY has an equivalent functional role to mammalian IgG, being responsible for general protective immune responses, but also mediating anaphylaxis usually associated with the IgE molecule in mammalian systems. Constant (C) and Variable (V) domains of the Heavy (H) and Light (L) chains of the antibodies are presented in different colors; complementarity determining regions are shown as red lines and interchain disulphide bonds as green lines; black circles indicate glycosylation sites. Fc (fragment crystallisable), Fab (fragment antigen-binding) and Fv (variable fragment) regions are indicated. B: Examples of monovalent antibody fragments that can be obtained by recombinant antibody engineering: Fab (50KDa), Fv (25KDa) and single-chain variable fragment, scFv (25KDa).
The Fc region of avian IgY has also diverged significantly from mammalian IgG and underlies a number of important features. The IgY-Fc does not bind to naturally occurring anti-mammalian antibodies, such as rheumatoid factor, nor to mammalian Fc receptor, and it does not activate the mammalian complement system. These features are in part responsible for the delayed anti-IgY response observed in pharmacokinetic studies performed on intravascular administration of IgY in rabbits27 and support the use of yolk antibodies in systemic neutralisation of venoms or toxins28,29. Additionally, IgY does not bind to standard immunoglobulin-binding resins, such as Protein-A (Staphylococcus aureus), Protein-G (Streptococcus sp.) or Protein–L (Peptostreptococcus magnus), and requires purification using an affinity column of the purified antigen.
A distinctive aspect of birds is their mechanism of immune diversification. Unlike mammals, they present a single functional VH or VL element in their heavy and light chain loci, respectively, and diversity is achieved by mechanisms of somatic hypermutation and gene conversion30. The latter involves incorporation of sequences from pseudo V-gene segments, which lack the transcription regulatory and signal-recognition sequences, into the functional V-elements. The organisation of the antibody genes in single functional elements simplifies the molecular cloning of avian immune repertoires and becomes an attractive feature for preparation of antibody libraries using in vitro display technologies such as phage display31. Immunised chickens, for example, can present an antigen-specific response in four to six weeks; at this stage their lymphoid organs such as Bursa of Fabricius, spleen and femur bone-marrow, harbouring antibody-producing B-cells, can be collected and processed to make cDNA (Figure 2). Libraries of recombinant avian antibody fragments can easily be generated using a single pair of cloning oligonucleotides for either light- and heavy-chain genes. This contrasts with the cloning of mammalian antibody libraries, which becomes rather complex due to the presence of more than one functional gene element per chain.
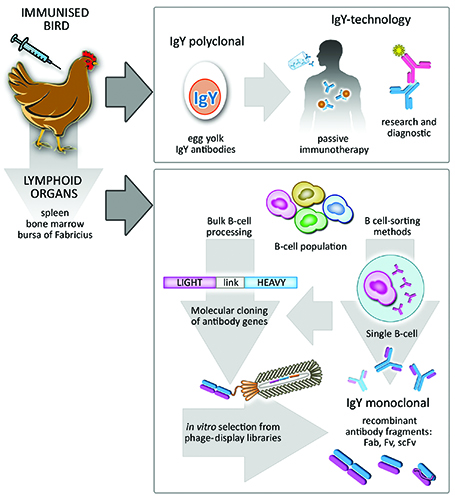

Figure 2: Overall potential of Avian IgY antibodies. Immunised birds naturally deposit protective antibodies in the yolk of their eggs. These are termed IgY antibodies, they are polyclonal and can be used in a vast number of passive immunotherapies in humans and animals as well as in research and diagnostic immunoassays. Furthermore, B-cell population recovered from avian lymphoid organs can be processed as bulk, leading to naïve or immune libraries of recombinant antibody fragments. Subsequent screening of antigen-specific monoclonal antibodies can be performed by standard phage-display methods. Alternatively, sophisticated single-cell sorting methods can be applied to the initial B-cell population in order to select B-cells producing an antibody of interest.
A number of antibody fragments can be generated of varying molecular size (Figure 1) and depending on the particular task required. The Fab fragment (~50kDa) for antigen recognition has been thoroughly explored as a stable molecule that tends to be monovalent in character. However, when recombinant Fab protein is expressed in E. coli their yields generally are very low. Currently, a commonly-used recombinant antibody fragment is the single-chain variable fragment (scFv) molecule (~25kDa) and when expressed or delivered inside a cell is called an intrabody. In this molecule the variable light (VL) and heavy (VH) domains of an antibody are linked by a flexible linker sequence (GS)n which should be long enough to allow these domains to interact. Both VH-VL or VL-VH configurations can be generated (Figure 1B) allowing scFv molecules to retain their ability to recognise specific antigens. The smaller size of the scFv molecule has an advantage in that higher yields can be attained from expression within the periplasm of E.coli. Most scFv molecules tend to multimerise; however this can be controlled by the length of the linker sequence between the variable domains and in some cases may be desired to enhance the avidity to the antigen target.
The availability of surface display vectors which allow the presentation of proteins on the surface of bacteria and filamentous bacteriophage by phage display32 has created a way to specifically select particular antibody properties during multiple cycles of screening and selection. Furthermore, selected positive antibody fragments can be matured for enhanced functions using random mutagenesis of the CDR regions and further phage selection rounds. The coupling of varying selection strategies during phage display screening with in vitro affinity maturation is a powerful technique to tailor specificity and affinity in the final antibody fragment.
In conclusion, immune and naïve antibody libraries derived from mammalian and avian sources are currently combined with sophisticated procedures, such as phage display screening, affinity maturation and molecular re-engineering of useful attributes, to enable the fine tuning of a robust antibody for a particular application. Importantly, fragment-based antibody technologies can easily be established in the research laboratory when used in combination with birds as an immune source. The distinctive immunological advantages of birds enables the generation of highly specific and unique antibodies, creating powerful research tools that allows the capture of distinct conformational protein states that can help to understand biological systems at the molecular level. The challenge for the future is to expand the creative use of antibodies to probe fundamental molecular mechanisms in biology, and birds are definitely going to play a part in this path.
Biographies




References
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015;7(1):9-14
- Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504(7478):101-6
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414(6859):43-8
- Uysal S, Cuello LG, Cortes DM, Koide S, Kossiakoff AA, Perozo E. Mechanism of activation gating in the full-length KcsA K+ channel. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(29):11896-9
- Uysal S, Vasquez V, Tereshko V, Esaki K, Fellouse FA, Sidhu SS, et al. Crystal structure of full-length KcsA in its closed conformation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6644-9
- Staus DP, Wingler LM, Strachan RT, Rasmussen SG, Pardon E, Ahn S, et al. Regulation of beta2-adrenergic receptor function by conformationally selective single-domain intrabodies. Molecular pharmacology. 2014;85(3):472-81
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nature medicine. 2004;10 Suppl:S2-9
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277(5334):1990-3
- Lecerf JM, Shirley TL, Zhu Q, Kazantsev A, Amersdorfer P, Housman DE, et al. Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4764-9
- Butler DC, Messer A. Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PloS one. 2011;6(12):e29199
- De Genst E, Chirgadze DY, Klein FA, Butler DC, Matak-Vinkovic D, Trottier Y, et al. Structure of a single-chain Fv bound to the 17 N-terminal residues of huntingtin provides insights into pathogenic amyloid formation and suppression. Journal of molecular biology. 2015;427(12):2166-78
- Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. Journal of molecular biology. 2007;368(4):1132-44
- Emadi S, Kasturirangan S, Wang MS, Schulz P, Sierks MR. Detecting morphologically distinct oligomeric forms of alpha-synuclein. The Journal of biological chemistry. 2009;284(17):11048-58
- Klemperer F. Über natürliche Immunität und ihre Verwertung für die Immunisierungstherapie. Arch für Exp Pathol und Pharmakologie. 1893;31:356-82
- Schade CS, C. Hendriksen, M. Erhard, H. Hugl, G. Koch, A. Larsson, W. Pollmann, M. van Regenmortel, E. Rijke, H. Spielmann, H. Steinbusch, and D. Straughan. The Production of Avian (Egg Yolk) Antibodies: IgY. Altern to Lab Anim (ATLA). 1996;24:925-34
- Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annual review of food science and technology. 2012;3:163-82
- Spillner E, Braren I, Greunke K, Seismann H, Blank S, du Plessis D. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals: journal of the International Association of Biological Standardization. 2012;40(5):313-22
- LaDeana W. Hillier et al. 2004;432:695-716
- Leslie GA, Martin LN. Studies on the secretory immunologic system of fowl. 3. Serum and secretory IgA of the chicken. Journal of immunology. 1973;110(1):1-9
- Kowalczyk K, Daiss J, Halpern J, Roth TF. Quantitation of maternal-fetal IgG transport in the chicken. Immunology. 1985;54(4):755-62
- Wang YW, Field CJ, Sim JS. Dietary polyunsaturated fatty acids alter lymphocyte subset proportion and proliferation, serum immunoglobulin G concentration, and immune tissue development in chicks. Poultry science. 2000;79(12):1741-8
- Carlander D. Avian IgY antibody: In vitro and in vivo. University of Uppsala; 2002
- Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunology today. 1995;16(8):392-8
- Parvari R, Avivi A, Lentner F, Ziv E, Tel-Or S, Burstein Y, et al. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain locus. The EMBO journal. 1988;7(3):739-44
- Wu L, Oficjalska K, Lambert M, Fennell BJ, Darmanin-Sheehan A, Ni Shuilleabhain D, et al. Fundamental characteristics of the immunoglobulin VH repertoire of chickens in comparison with those of humans, mice, and camelids. Journal of immunology. 2012;188(1):322-33
- Conroy PJ, Law RH, Gilgunn S, Hearty S, Caradoc-Davies TT, Lloyd G, et al. Reconciling the structural attributes of avian antibodies. The Journal of biological chemistry. 2014;289(22):15384-92
- Diaz P, Malave C, Zerpa N, Vazquez H, D’Suze G, Montero Y, et al. IgY pharmacokinetics in rabbits: implications for IgY use as antivenoms. Toxicon: official journal of the International Society on Toxinology. 2014;90:124-33
- LeClaire RD, Hunt RE, Bavari S. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infection and immunity. 2002;70(5):2278-81
- Meenatchisundaram S, Parameswari G, Michael A, Ramalingam S. Studies on pharmacological effects of Russell’s viper and Saw-scaled viper venom and its neutralization by chicken egg yolk antibodies. International immunopharmacology. 2008;8(8):1067-73
- Reynaud CA, Dahan A, Anquez V, Weill JC. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59(1):171-83
- Rader C, Barbas CF, 3rd. Phage display of combinatorial antibody libraries. Current opinion in biotechnology. 1997;8(4):503-8
- Andris-Widhopf J, Rader C, Steinberger P, Fuller R, Barbas CF, 3rd. Methods for the generation of chicken monoclonal antibody fragments by phage display. Journal of immunological methods. 2000;242(1-2):159-81
Related topics
Antibodies, Biologics, Immunotherapy, Protein, Technology, Therapeutics