The promise and pitfalls of serial femtosecond X-ray crystallography
Posted: 4 September 2019 | Victoria Rees (Drug Target Review) | No comments yet
A report from scientists at the Moscow Institute of Physics and Technology highlights the advantages and disadvantages of serial femtosecond X-ray crystallography. This article investigates the review, focusing on its role in drug development.

Crystallography is a vital technique used in drug discovery and development, which is often evolving and improving. Researchers from the Moscow Institute of Physics and Technology (MIPT), Russia published a review on serial femtosecond X-ray crystallography (SFX), highlighting the technology’s advantages and disadvantages.1
The article delves into the reasons behind the researcher’s findings, explaining why SFX is a progression for X-ray imaging in drug development, while highighting that its application in structure-based drug discovery (SBDD) requires further changes to reduce the time and costs associated with the technique.
Dr Alexey Mishin, deputy head of the Laboratory for Structural Biology of Receptors at MIPT, who co-authored the study, explains: “[what makes it a] breakthrough technology is a very high-energy density of the laser pulse. The object is exposed to such powerful radiation that it falls apart, inevitably and almost instantly. But before it does, some individual quanta of the laser pulse scatter off the sample and end up at the detector. This is the so-called diffraction-before-destruction principle for studying the structure of the original protein.”
X-ray crystallography

Firstly, the protein is isolated and purified, leaving the solvent to gradually dry out. This causes the molecules to form crystals, characterised by an internal order. By exposing a crystal to X-rays, researchers can obtain a diffraction pattern that contains information regarding the positions of atoms within the crystal.
This method of imaging allows scientists to view 3D structures as computational models and programmes can quickly sort through millions of potential compounds to find drug candidates. This saves the essential resources of both time and money.
The authors say that X-ray crystallography produces results for crystals that are large, stable and homogenous. To better detect a weak diffraction signal, a powerful radiation pulse is needed; but not so strong as to destroy the crystal. In conventional X-ray crystallography, a protein crystal is rotated in the X-ray beam to produce diffraction patterns for various spatial orientations to capture maximum information on the structure.
Why use SFX?
The review explains that soon after X-ray crystallography emerged, it became evident that not all biological macromolecules could be crystallised. As some proteins are ordinarily dissolved in the inner cell medium, it is relatively easy to put them into solution and evaporate it, resulting in a large regular crystal.
However, membrane proteins form crystals that are not large and pure enough for standard X-ray crystallography. Many of these proteins are involved in disease development, so obtaining their structure is important for researchers, says the report, highlighting the value of the SFX method in drug discovery.
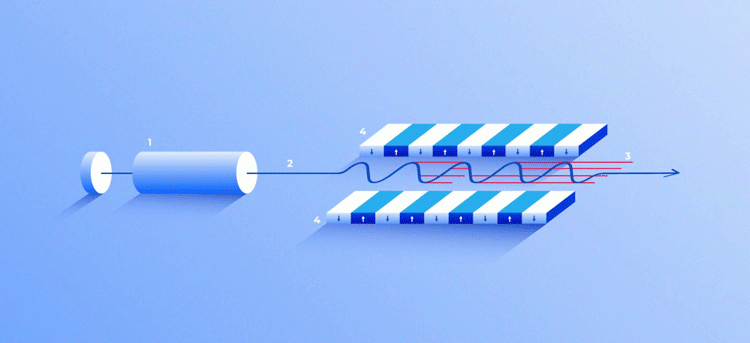

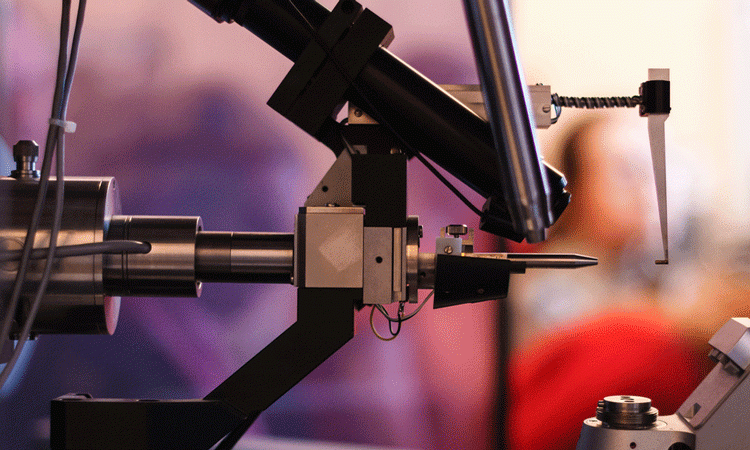
X-ray free-electron laser. A source (1) emits free electrons (2) moving merely tens of times slower than the speed of light through the undulator (4), a tunnel lined with many magnets. The magnetic field causes an electron traveling through the tunnel to oscillate and therefore emit X-rays. The movement of the electrons in the undulator is synchronised to generate tight, high-frequency X-ray pulses of remarkable intensity (3) [credit: Elena Khavina/MIPT Press Office].
Mishin explains that SFX is a relatively novel data collection method in crystallography, where data are typically collected from thousands of crystals sequentially delivered into the focal point of the X-Ray Free Electron Laser (XFEL) beam in random orientations.
He points out that although the “XFEL beam is made of extremely bright femtosecond-range pulses that can annihilate small crystals,” it is still possible to obtain the diffraction pattern before this destruction happens.
Therefore, according to Mishin’s report, the SFX technique provides a solution with which to study these membrane proteins.
The advantages of SFX
Mishin explains that SFX is a useful tool to purify or crystallise membrane and soluble proteins. In regular X-ray crystallography, crystals are often too small and their diffraction resolution can be of poor quality.
He expects that remote, automated experiments will be a common practice, especially for fragment and compound screening”
The emergence of XFELs about a decade ago introduced the possibility of overcoming radiation damage and enabling high-resolution, room-temperature structure determination from very small or heterogeneous crystals, he says. Most importantly, it also meant “it provided access to studying conformational transitions in proteins with a sub-picosecond time resolution.”
According to Mishin and the researchers, the unique qualities of the SFX technique therefore make it an attractive option for the study of small crystals. This can then be applied to the investigation of the mechanisms behind drug targets, such as structure-activity relationships.
What are the disadvantages of SFX?
Despite this, the review highlights that conventional X-ray crystallography involves exposing one crystal to radiation from various angles and analysing the resulting diffraction patterns collectively; whereas, in SFX, the crystal is instantly destroyed by the first interaction with a powerful X-ray pulse. This means that researchers must repeat the process with many small crystals and analyse the “serial” data generated.
…at XFELs only a few stations are connected to the hard X-ray tunnel”
A further challenge with SFX identified in the report, is selecting appropriate samples. In the case of the conventional technique, choosing the largest and highest-quality crystal could be done manually, through visual inspection of the sample. However, the new procedure requires working with a suspension of many small crystals of varying size and quality. Centrifuges and filters with known pore dimensions are used to separate the crystals by size, which makes the process more complex, according to the report.
The method used for placing samples into the chamber needed changing too, remarks the review. X-ray free-electron lasers have a maximum frequency at which they can emit radiation pulses. To reduce expense and time consumption, new crystals must be fed into the chamber at the same frequency.
Thus far, two approaches have been developed:
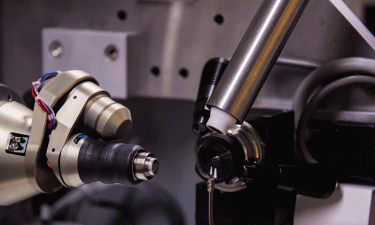
- The crystals enter the chamber in a liquid suspension, supplied by an injector. The jet leaving the injector is compressed by a stream of gas to ensure correct sample placement; so, when passing through, a crystal ends up precisely at the centre of the laser beam
- The protein crystals can be spread over a substrate transparent to X-rays and automatically fed into the laser beam before each pulse.
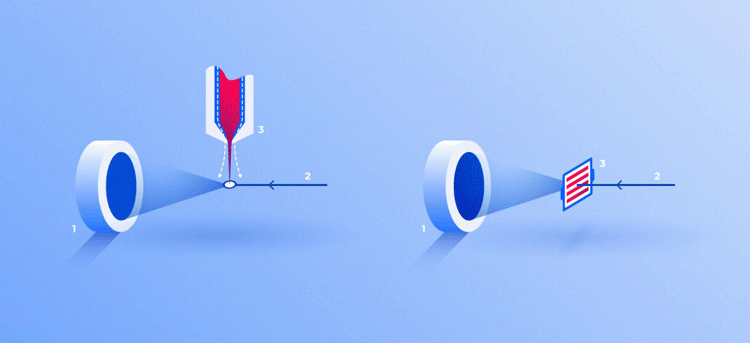

Two ways for feeding crystals into the operating area of the device: in a stream of liquid (3, left) and on a solid substrate (3, right). In both cases, the X-ray beam (2) passing through crystals generates a diffraction pattern on the screen (1) [credit: Elena Khavina/MIPT Press Office].
However, Mishin comments that the most important drawback of the technology “is low availability of the instruments for users. At modern synchrotrons, multiple multifocus beamlines are usually available simultaneously for several user groups, but at XFELs only a few stations are connected to the hard X-ray tunnel.” As only one station can work at a time, switching between them can cause time delays for beam calibration.
Therefore, although SFX is needed to investigate membrane proteins to improve drug development, it is not a perfect technique and could be improved.
The future of SFX
Mishin hopes that in the future there will be “more SFX instruments available worldwide, even including compact in-home facilities.” He expects that remote, automated experiments will be a common practice, especially for fragment and compound screening.
He suggests areas of improvement needed for the technology, such as the “XFEL light source, X-ray focusing optics, sample delivery setups, advanced high-gain detectors with high time resolution and special data treatment software and algorithms.”
Mishin says that this is necessary “in order to understand specific target activation and working cycles; eg, triggering with photoswitchable compounds and analysis of ligand-binding kinetics and dynamics,” as this could be important for drug discovery.
Conclusion
Since producing its first results in 2011, SFX has revealed over 200 protein structures. These include 51 targets potentially important for pharmacology, which used to be inaccessible for conventional analytical techniques.2
Since producing its first results in 2011, SFX has revealed over 200 protein structures”
Mishin says that the most exciting drug discovery findings resulting from this technique are the “discovery of G Protein Coupled Receptors (GPCRs) and their complexes with G proteins and arrestin; the crystal structure of the BEV2 virus with a sphingosine molecule and molecular movies of retinal isomerisation in bacteriorhodopsin.”
Therefore, according to the researchers, this “cutting-edge” technique is highly important for drug discovery. Although there are some drawbacks, it opens up the possibility for many new discoveries in the development of drugs.
The findings were published in Expert Opinion of Drug Discovery.
References
- Mishin A, Gusach A, Luginina A, Marin E, Borshchevskiy V, Cherezov V. An outlook on using serial femtosecond crystallography in drug discovery [Internet]. Taylor & Francis. 2019 [cited 29 August 2019]. Available from: https://www.tandfonline.com/doi/full/10.1080/17460441.2019.1626822
- X-ray laser sight reveals drug targets — Moscow Institute of Physics and Technology [Internet]. Mipt.ru. 2019 [cited 29 August 2019]. Available from: https://mipt.ru/english/news/x_ray_laser_sight_reveals_drug_targets
Related topics
Crystallography, Drug Targets, Hit-to-Lead, Imaging, Research & Development, X-ray Crystallography
Related organisations
Moscow Institute of Physics and Technology (MIPT)
Related people
Dr Alexey Mishin