Developing respiratory therapeutics for prophylaxis and treatment
Posted: 30 April 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Drug Target Review explores antiviral Fc-conjugates and how they could be used as a COVID-19 prophylactic and therapeutic with Dr Jeff Stein, Cidara’s President and CEO.

With the scale of the COVID-19 pandemic still growing, many pharmaceutical companies are adapting their current R&D programmes to develop therapeutics or vaccines to help stop the spread of the SARS-CoV-2 coronavirus causing the outbreak. Drug Target Review’s Hannah Balfour explores how Cidara is transferring its expertise in therapeutics for respiratory viruses to focus on developing a potential treatment for COVID-19 with its President and Chief Executive Officer, Dr Jeff Stein.
According to Stein, Cidara’s original aim was to apply the principles from bispecific immunotherapies, which have revolutionised the treatment of cancer patients, to the treatment and prevention of infectious diseases such as fungal, bacterial and viral infections. The platform the company bases its therapeutic development on is called the Cloudbreak® Antiviral Fc-Conjugate (AVC) Platform.
Cidara’s more advanced programme is an antifungal drug called Rezafungin which is currently in Phase III trials for candidemia and invasive candidiasis and licenced to Mundipharma outside of the US and Japan. Stein explained that the Cloudbreak platform has evolved from developing anti-fungal drugs to bacterial and now to viral. Through these developments, they discovered that the properties of the drug products, called AVCs, “lend themselves quite well to respiratory viruses”.
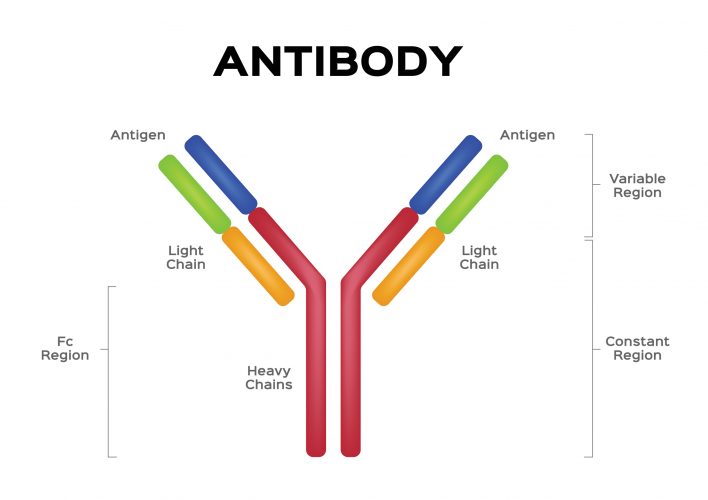

AVCs are fusion molecules, designed using similar principles as bispecific antibodies for immuno-oncology. The molecule consists of the Fc region of a human antibody that acts as an effector domain, engaging the patient’s immune system to accelerate the elimination of the pathogen, fused with a targeting domain (small molecule or peptide) that neutralises a virus by directly binding to it and preventing viral proliferation through a variety of potential mechanisms. Stein stated that because of their two distinct mechanisms of action, AVCs can be used both as a prophylactic agent – to prevent a viral infection – or as a therapeutic – to cure an infection.
Antiviral Fc-conjugates and influenza
Their most advanced viral infection programme targets influenza – CD377 is designed for the treatment and seasonal prevention of the virus. According to Stein this AVC may have several benefits over traditional vaccine strategies, including its potency and ability to suppress the influenza virus even in immunocompromised patients that do not respond to vaccines, based on efficacy studies conducted in severely immune compromised animal models.
…CD377 has the ability to both cure active infections and induce an immune response that may enable it to be used as a substitute for a universal seasonal flu vaccine”
CD377 uses an engineered version of a highly potent neuraminidase enzyme inhibitor, an antiviral small molecule drug for treatment or postexposure prophylaxis of influenza, as its targeting domain. Stein said that despite its potency, this molecule alone has poor drug properties – pharmacokinetics that require the drug to be delivered by inhalation and undesirable side effects – and was ostensibly overtaken by Tamiflu due to these characteristics. Stein continued that Tamiflu, an oral antiviral medication for the same indication, also has other liabilities such as limited spectrum, increased resistance and it must be administered with 48 hours of the initial symptoms of the flu to be effective.
He said that by “engineering this neuraminidase inhibitor, one of the world’s best enzyme inhibitors, and attaching multiple copies of it to the Fc fragment of a human antibody we have improved its drug properties, including its pharmacokinetics, which may provide seasonal prevention with one dose. In pre-clinical animal experiments, our small molecule AVCs distributed to the lungs within minutes, regardless of the route of administration. We have administered them by intravenous infusion (IV), by subcutaneous injection and by intramuscular injection and found in all cases the AVCs get to the lungs very rapidly and therefore are an ideal approach for respiratory viruses.”
Stein commented that, “researchers are currently testing CD377 in animal models in collaboration with the US National Institutes of Health (NIH). Their feedback is that this is among the most potent influenza drugs they have tested to date and they have requested additional materials so they can do more extensive animal testing with it.” In other experiments, CD377 has the ability to both cure active infections and induce an immune response that may enable it to be used as a substitute for a universal seasonal flu vaccine, as it demonstrated protection against all known strains of influenza, including influenza A and B, seasonal and pandemic strains.
The future for antiviral Fc-conjugates
HIV, respiratory syncytial virus (RSV) and COVID-19
Alongside its influenza programmes, the company has also expanded its research into human immunodeficiency virus (HIV), respiratory syncytial virus (RSV) and more recently the SARS-CoV-2 pathogen responsible for the COVID-19 outbreak.
…Cidara is developing a series of AVCs for coronaviruses, including some with pan-SARS activity… and others with a more potent, focused activity against SARS-CoV-2 subtypes”
To target these viruses, Cidara either uses existing small molecule or peptide fusion inhibitors, as was the case for SARS-CoV-2, or uses the viral DNA sequence to design novel inhibitors to use as the targeting domain (antiviral) in the AVCs. These are then fused to the Fc backbone to create an AVC molecule. Stein said that the pharmacokinetic properties provided by the AVC platform suggest that COVID-19 candidates have the potential to be a very fast-acting treatment and a long-acting preventative, as is true of the influenza, HIV and RSV programmes.
According to Stein, Cidara is developing a series of AVCs for coronaviruses, including some with pan-SARS activity – ie, designed to be effective against the entire SARS family of coronaviruses, including SARS-CoV, SARS-CoV-2 and MERS, alongside others being designed to have a more potent, focused activity against SARS-CoV-2 subtypes.
Challenges for AVC development
Deciding a dosage
Stein explained that stumbling blocks typically occur during pre-clinical drug development, such as a weakness in the product that may limit its dosage or route of administration. “For example, the neuraminidase inhibitor we studied has poor pharmacokinetics and distribution in vivo so must be delivered by inhalation to ensure enough of the product reaches the target to be effective. Others may have a small therapeutic index, meaning the difference between the therapeutic dose and the toxic dose is small, so there are limited dosage options and a possibility of side effects.
“Our problem is that our CD377 influenza programme has not presented one of these problems, which can challenge the process of deciding the indication for clinical testing. It is incredibly potent in vivo and we have tested it in both rodent and primate toxicity models at doses up to 54-fold higher than the effective dose and have seen no side effects. We basically have not found a toxic dose of this drug.”


He continued: “there will probably be two different doses of the drug – one for treatment and one for prevention – and possibly different formulations for these uses. For use as a therapeutic, its potency and ability to concentrate in the lung, and need for a shorter duration or action, mean a smaller dose can be used, possibly administered through IV infusion for severely ill hospitalised patients or subcutaneously if an outpatient. To use the drug as a prophylactic seasonal flu injection would require a dose about three times that of the therapeutic dose, it could be administered either subcutaneously or through intramuscular injection, like current vaccines.”
R&D
Stein explained that the R&D phase of the influenza programme went relatively swiftly because of the existing animal models, accessible through various universities, contract research organisations (CROs) as well as Cidara’s and government labs. However, in vivo and in vitro models for SARS-CoV-2 are currently being developed and are in extremely high demand: “this is the most challenging aspect of developing therapies for the pandemic.”
He continued that the earliest experts expect these models to be available is May 2020, so development and testing will be slower for the COVID-19 programme.
Conclusion
Overall, Stein described a drug platform with high adaptability, able to identify AVC’s with great pharmacokinetic properties that are well suited to treating and preventing various respiratory viruses, including SARS-CoV-2. He said that the company needs to conduct further pre-clinical testing of its COVID-19 AVC programmes before entering clinical trials. However, utilising the various regulatory support opportunities being established for drugs related to COVID-19 may speed the time frame of delivering this drug for patient use, once the pre-clinical phase is complete.
He concluded that the development of COVID-19 AVCs has benefited from the learnings of the CD377 programme for influenza and could potentially provide both a prophylactic and therapeutic strategy for COVID-19, where there is currently none.
Related topics
Antibodies, Antifungal Therapies, Bioengineering, Disease Research, Drug Development, Immunology, In Vitro, In Vivo, Research & Development, Therapeutics, Vaccine
Related conditions
Candidiasis, Coronavirus, Covid-19, fungal infections, HIV, Influenza, respiratory syncytial virus (RSV)
Related organisations
Cidara Therapeutics, Mundipharma, US National Institutes of Health (NIH)
Related people
Dr Jeff Stein