Oncology meets immunology – antibody-mediated regulation of the immune response
Posted: 10 September 2020 | Aparajita Dubey (CRAMbridge) | No comments yet
In this article, Aparajita Dubey discusses the role of antibodies in regulating the immune system and highlights key features that need to be considered for drug development and how this can be applied to cancer therapy.

The use of antibodies to restore balance in the immune system is based on an old phenomenon called antibody-mediated feedback regulation. The premise is that where antibodies are administered together with a specific antigen in vivo they can regulate the immune response to that antigen. Autoimmunity typically occurs when there is dysfunction or imbalance in the immune system due to a failure in normal tolerance mechanisms. Tolerance mechanisms should ensure that T and B cells are reactive enough to prevent infections, but not autoreactive, ie, do not stimulate the immune system in response to ‘self’ or ‘auto’ antigens. These mechanisms have been studied and the research has given rise to a number of monoclonal antibodies (mAbs) and genetically engineered biological agents targeting the immune system as treatments for a range of autoimmune disorders. However, despite these advancements, effective therapies for other autoimmune conditions (such as type 1 diabetes) remain questionable and will likely require combinatorial interventions targeting multiple components of the immune system.
The discovery of mAb production by Kolher and Milstein revolutionised the field of antibody engineering and targeted therapeutic agents. As their high specificity results in fewer adverse effects, therapeutic antibodies have become the predominant class of drugs being developed, with fully human mAbs being the most promising category of targeted therapeutic agents. Furthermore, to overcome some of the limitations associated with mAb therapy, several mAb-based modalities are under development, including antibody drug conjugates (ADCs), fusion proteins and bispecific antibodies (bsAbs).
To overcome some of the limitations associated with mAb therapy, several mAb-based modalities are under development”
Based on the clinical success of immunomodulatory antibodies, novel antibody therapies for cancer now include ADCs for lymphomas and solid tumours. What is more, a large number of immune checkpoint receptors and ligands have been identified and are being investigated as potential contributors to the pathogenesis of autoimmune diseases and as targets for effective therapeutic interventions. To achieve this, several approaches that manipulate cells ex vivo and harness their complex behaviours are being tested in pre-clinical and clinical settings. Approved biologic agents are also being examined in combination and with cell-based therapies. However, various development and regulatory hurdles must be overcome to successfully combine immunotherapeutic biologic agents to ensure autoimmune disease manifestations are controlled and the tolerant state is restored.
Antibody function and key features of the immune system
Antibodies are secreted into the blood and mucosa where they are involved in neutralisation, ie, inactivation of foreign substances such as pathogens and toxins. They activate the complement system to destroy bacterial cells by lysis and facilitate phagocytosis of foreign substances by phagocytic cells through a process called opsonisation. Antibody-dependent cellular cytotoxicity (ADCC) is another major antibody function. Key features of antibodies and the immune system that need to be considered for drug development include:
- The specificity of antibodies – antibodies react with some antigenic determinants and not with others. This is dependent on chemical composition, physical forces and molecular structure at the binding site.
- The diversity of antibodies – antibodies are encoded by different germ-line genetic loci. Antibody diversity is generated from the large number of variable (V), joining (J), diversity (D) and constant (C) genes available for recombination.
- Immunological memory – this is the ability of the immune system to quickly and specifically initiate a corresponding immune response after recognising an antigen that the body has previously encountered. Generally, these are secondary, tertiary and other subsequent immune responses to the same antigen.
- Immune tolerance – a state of unresponsiveness to a specific antigen or group of antigens to which a person is normally responsive. Immune tolerance occurs under conditions that suppress the immune reaction; it is not just the absence of an immune response.
Autoimmunity and autoantibodies
Inappropriate activation of T cells, B cells or both can lead to autoimmunity, resulting in damage to one or more organ system. Central immune tolerance is a process that normally eliminates high-affinity self-reactive T and B cells in the thymus and bone marrow. However, complex and non‑redundant peripheral tolerance mechanisms keep low-affinity self-reactive T and B cells, which escape central tolerance and enter the blood and tissue, in check.
Natural antibodies that react with self-molecules can arise in healthy individuals. They provide a first-line defence against infections and contribute to the homeostasis of the immune system. These natural autoantibodies are mainly of the immunoglobulin M (IgM) serotype, encoded by unmutated V(D)J genes, and display a moderate affinity for self‑antigens. They also typically inhibit autoimmune inflammation and enhance apoptotic cell removal. In contrast, high-affinity, somatically mutated immunoglobulin G (IgG) autoantibodies can disturb homeostatic pathways involved in cell clearance, antigen-receptor signalling or cell effector functions. These IgG autoantibodies are pathogenic and implicated in autoimmune disorders.
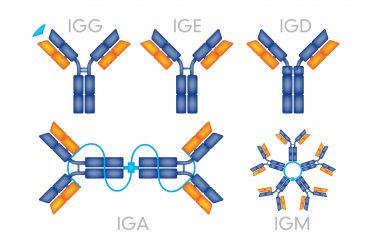

Different antibody serotypes and their structures.
In some systemic autoimmune disorders, autoantibodies are present before disease onset with remarkable specificity and can serve as biomarkers. However, in organ-specific autoimmune diseases, such as myasthenia gravis or pemphigus, where autoantibodies directly bind to and injure the target organs, this is less common. In systemic autoimmune diseases, autoantibodies form pathogenic antigen–antibody (immune) complexes by reacting with free molecules, such as phospholipids, as well as cell surface and nucleoprotein antigens. Engagement and activation of FcγR, activation of the complement system or internalisation and activation of Toll‑like receptors leads to the production of type I interferon and other inflammatory cytokines that amplify autoinflammatory responses.
Therapy for autoimmune disease
Autoimmune therapies seek to restore the immune system to a tolerant state where there is sufficient reactivity to avoid infection and cancer, but autoimmune reactivity is minimised. The balance of the immune system is regulated by underlying mechanisms involving co‑stimulatory and co‑inhibitory receptors and other molecules that function as immune checkpoints in autoimmune diseases such as systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis (RA), Sjögren’s syndrome, type 1 diabetes and inflammatory bowel disease. The discovery of novel immune checkpoint receptors and ligands during the last decade has revolutionised the treatment of autoimmune diseases. As a result, biologics including mAbs and engineered fusion proteins have emerged as standard treatments in autoimmunity, especially when traditional disease‑modifying drugs fail to control the disease. Several biologics are US Food and Drug Administration (FDA)-approved for the treatment of rheumatoid arthritis, psoriasis and inflammatory bowel disease. However, the clinical trials of agents targeting these checkpoint pathways in autoimmune diseases have also highlighted some possible risks and challenges.
Approved biologic agents are also being examined in combination and with cell-based therapies”
Several new biologic agents are currently under development and the variety of mechanisms they employ include cytokine blockade, depletion of T or B cells and immunomodulation. Several small molecules also target the immune response. However, the complexities of autoimmune disease pathogenesis mean that many specific biologic agents are associated with negative consequences, such as vulnerability to infection, because they compromise the normal immune response. Moreover, none of the biologics have been shown to restore immune tolerance to the extent that disease remains dormant after therapy is withdrawn. In many cases, biologic agents are unable to fully control autoimmunity; for example, treatment of type 1 diabetes with the anti-CD3 antibodies teplizumab or otelixizumab, the anti‑CD20 mAb rituximab or the CTLA-4 fusion protein abatacept did not achieve sufficient tolerance. In each case most participants experienced a recurrence of progressive β-cell loss. Several new therapeutic strategies for treating type 1 diabetes are also being considered, including biologic agents that target the cytokines IL-1, TNF, IL-12p40, IL-17 and IL-6.
Combination therapy for autoimmune disease
Combinational therapy is important for increasing efficacy in autoimmune disease treatment because numerous non-redundant mechanisms contribute to the maintenance of normal immune tolerance. Complementary and non-redundant pathways can be targeted in sequence to first induce autoimmune disease remission then restore a state of immune tolerance to prevent recurrence of the disease. A detrimental cascade of events occurs upon activation of autoimmune processes, leading to downstream activation of pathogenic cell populations and the production of pro-inflammatory cytokines. mAbs and recombinant fusion proteins that target pro‑inflammatory cytokines such as TNF, IL-1, IL-6, IL- 12 and IL-23 are extremely effective for some autoimmune indications and agents that target IL-17 are also under development. Combination therapy can also solve the problem of recurrent autoimmune manifestations upon removal of biologic agents if the second biologic agent induces tolerance in effector cells undergoing reactivation or reconstitution.
Interactions between cancer and the immune system
To a certain extent, the immune system can recognise and eliminate aberrant cancer cells arising within the human body”
The composition of the innate and adaptive immune system allows it to detect a wide variety of infectious organisms and other invaders, distinguish them from the body’s own healthy cells and tissues and prevent infections. The cancer immunosurveillance theory refers to the monitoring function of the immune system in cancer development. To a certain extent, the immune system can recognise and eliminate aberrant cancer cells arising within the human body; however, in cancer patients the antitumour immune system is suppressed and not sufficiently vigorous to eliminate cancer cells. This principle is demonstrated by transplant recipients under continued immunosuppression, who have a higher risk of developing de novo tumours such as lung cancer and cervical cancer. There are various factors that may contribute to antitumour immunosuppression, including a low frequency of high-avidity antitumour T cells, presence of CD4+ and CD25+ regulatory T (Treg) cells, as well as various strategies of cellular mediated tumour-induced immune evasion. It may also result from soluble factors and altered antigenicity, eg, tumour-derived IL-18 in NK cells, IL-1a upregulated TGF-beta in mesenchymal stem cells, leading to the growth of prostate cancer cells. Hence, immunological methods that eliminate antitumour immunosuppression or increase antitumour immunity can be a useful means in treating cancer.
Antibody-based therapy for cancer
Antibody therapies have been established as the most effective strategy for treating patients with haematological malignancies and solid tumours for the past 15 years. In order to identify candidate mAbs, researchers must undertake complex scientific and pre-clinical evaluations. These include the identification of the physical and chemical properties of the antibody, detailed specificity analyses of antigen expression, studies of the immune effector functions and signalling pathway effects, analyses of in vivo antibody localisation and distribution in transplanted or syngeneic tumour systems, along with observation of the in vivo therapeutic activity of the antibody. Clinical evaluation of the antibody plays a crucial role in determining its toxicity and therapeutic efficacy alone or as a delivery system for radioisotopes or other toxic agents. In vivo specificity is evaluated by determining its bio‑distribution in patients and using this to assess the ratio of antibody uptake in the tumour versus normal tissues.
Antibody-based therapies employ the following mechanisms to kill tumour cells:
a) Direct tumour cell killing: This can be elicited or mediated by receptor agonist activity. In one case, an antibody may bind to a tumour cell-surface receptor and activate it to initiate apoptosis, where other examples block dimerisation, kinase activation and downstream signalling, leading to reduced proliferation and apoptosis. An antibody binding to an enzyme can lead to neutralisation, signalling abrogation and cell death, whereas conjugated antibodies can be used to deliver multiple payloads (such as a drug, toxin, small interfering RNA or radioisotope) to a tumour cell.
b) Immune-mediated tumour distruction: Induction of phagocytosis, complement activation and ADCC can result in cancer cell death. T cells are being targeted or activated to destroy tumours using three methods: single-chain variable fragment (scFv), antibody-mediated cross‑presentation of antigen to dendritic cells or by inhibition of T-cell inhibitory receptors, such as cytotoxic T lymphocyte-associated antigen 4 (CTLA4).
c) Vascular and stromal cell ablation: Vasculature receptor antagonism or ligand trapping, stromal cell inhibition, delivery of a toxin to stromal cells and delivery of a toxin to the vasculature can all cause cancer cells to die off.
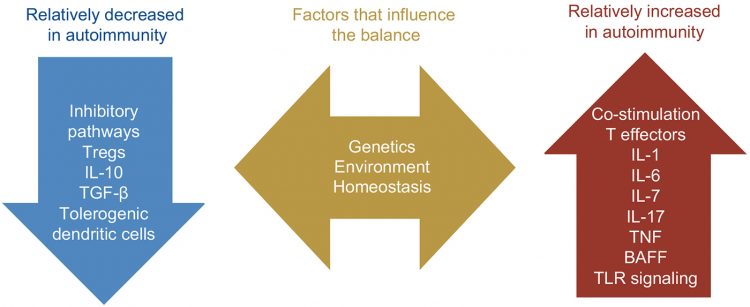

Figure 1: Mechanisms of tumour cell killing by antibodies.
Cancer immunity cycle and cancer immunotherapy
Immunity generation is a self-propagating, cyclic process that aims to amplify and broaden T-cell responses by accumulation of immune-stimulatory factors. Immunity development can be limited or checked by inhibitory factors that lead to immune regulatory feedback mechanisms. The cancer immunity cycle can be divided into seven major steps, initiated with the release of antigens from the cancer cell and ending with the killing of cancer cells.
Cancer progression is caused by genetic and cellular alterations. These also guide the immune system to generate T cells that recognise and eradicate cancer cells. Elimination of cancer by T cells manages the delicate balance between the recognition of non-self and the prevention of autoimmunity. A new class of cancer immunotherapy emerged with the identification of cancer cell T cell inhibitory signals, like PD‑L1 (which specifically hinders immune effector inhibition). These treatments strengthen and expand pre‑existing anticancer immune responses. Suppressive factors present in the tumour microenvironment play a major role in making immune-based therapies more effective, particularly in combination with agents that target other steps of the cycle.


CAR T cell receptor binding to cell surface protein.
Our understanding of immune tolerance will be foundational for new approaches to cancer immunotherapy, as these depend on overcoming multiple mechanisms that mediate immune tolerance to self-antigens. Adoptive transfer of immune effectors such as antitumour mAb and chimeric antigen receptor (CAR) T cells allows expansion of tumour-specific effectors ex vivo. Vaccination with whole tumour cells, protein, peptide or dendritic cells in combination with other cancer immunotherapeutic strategies may present a better option.
An immunomodulatory approach to cancer immunotherapy involves treatment with agents that enhance and maintain T-cell activation. Use of the checkpoint blockade to block negative signals and maintain antitumour responses are some of the recent advancements in the field and it is likely that cancer immunotherapy designed to overcome immune tolerance will be used to treat a growing number of cancer patients.
Recent market trends in the development of therapeutic antibodies
Due to active development and approval of new therapeutic antibody drugs for the treatment of various human diseases, including many cancers, autoimmunity, metabolic and infectious diseases, there is exponential growth in the market. In 2018, eight of the top 10 bestselling drugs worldwide were biologics and as a result the global therapeutic mAb market was valued at approximately $115.2 billion. A total of 79 therapeutic mAbs were approved by the FDA by December 2019, with significant potential for this to increase in the future. Therefore, the market is expected to generate $300 billion in revenue by 2025.
Conclusions
Autoimmunity is a challenge for all clinicians; however, there has been a dramatic improvement in the prognosis for patients with these diseases. While several disease-modifying drugs and biologics have been developed for the treatment of different autoimmune conditions, there are some limitations associated with these approaches in terms of durability of the therapeutic responses and requirement for long-term continuation of therapy. Most subjects with type 1 diabetes, when treated with immunomodulation, have shown a transient rather than sustained response. Immunosuppression remains the standard of care for other conditions, such as lupus nephritis, despite the associated toxicity. A substantial effort is required to fulfil the unmet medical needs for these conditions, which could be achieved by successful restoration of immune tolerance involving multiple immunological pathways.
The success of antibody therapeutics in cancer is due to our increased understanding of the complexities of antibody serology, target selection, antibody–receptor function and immune regulation of tumour growth. Antibody therapies for cancer can be progressed further by applying innovative approaches to target and antibody selection and by designing appropriate development strategies based on early-phase clinical trials.
About the author
Aparajita Dubey has more than 12 years of experience in pharmaceutical research, mainly in cancer biology and immunotherapy, at Biocon, Panacea and Jawaharlal Nehru University. As a biologist, she has experience in the fields of cell-line development, cancer immunotherapy and molecular biology. She has also worked as a medical writer and consultant and currently works as a content writer at CRAMbridge. She has a MSc in Life Sciences and a PG Diploma in IP from National Law School University, Bangalore, India.
Related topics
Antibodies, Disease Research, Drug Development, Drug Targets, Immunology, Immunotherapy, Oncology
Related conditions
autoimmune diseases, Cancer, Inflammatory bowel disease (IBD), Multiple Sclerosis (MS), Myasthenia gravis, Pemphigus, Psoriasis, rheumatoid arthritis, Sjögren's Syndrome, Systemic Lupus Erythematosus, type 1 diabetes
Related organisations
Fluidic Analytics, ForteBio








