New research points to a possible driver of Parkinson’s disease
Posted: 28 February 2022 | Ria Kakkad (Drug Target Review) | No comments yet
A study has shown how Parkinson’s disease may be driven by cell stress-related biochemical events that disrupt a key cellular clean-up system.


A new study by Scripps Research, US highlights that Parkinson’s disease may be driven in part by cell stress-related biochemical events that disrupt a key cellular clean-up system, leading to the spread of harmful protein aggregates in the brain. According to the researchers, the breakthrough, outlined in The Journal of Neuroscience, provides a clear and testable hypothesis about the progression of Parkinson’s disease and may lead to treatments capable of significantly slowing or even stopping it.
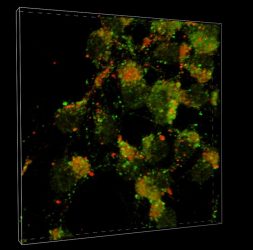

A team at Scripps Research reveals details into how Parkinson’s disease and Lewy body dementia spread in the brain. In neurons, the LC3 protein (green) and LAMP1 protein (red) fuse together into autolysomes (yellow) where autophagy, a cellular mechanism for clearing misfolded proteins, occurs. The prominence of green shows that autophagy has been blocked at the fusion step, allowing misfolded proteins like alpha-synucelin to instead spread throughout the brain.
[Credit: Scripps Research]
Parkinson’s precise trigger is unknown, but it entails the deaths of neurons in a characteristic sequence through key brain regions. The killing of one small set of dopamine-producing neurons in the midbrain leads to the classic Parkinsonian tremor and other movement impairments. Harm to other brain regions results in various other disease signs including dementia in late stages of Parkinson’s. A closely related syndrome in which dementia occurs early in the disease course is called Lewy Body Dementia (LBD). In both diseases, affected neurons contain abnormal protein aggregations, known as Lewy bodies, whose predominant ingredient is a protein called alpha-synuclein. Prior studies have shown that alpha-synuclein aggregates can spread from neuron to neuron in Parkinson’s and LBD, apparently transmitting the disease process through the brain. However, precisely how alpha-synuclein aggregates build up and spread in this way has been unclear.
Prior studies conducted by Scripps Research found that that the Parkinson’s/LBD disease process generates highly reactive nitrogen-containing molecules including nitric oxide. In principle, these reactive nitrogen molecules could disrupt important cellular systems, including “housekeeping” systems that normally keep protein aggregates under control.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
In the new study, the team demonstrated the validity of this idea by showing that a type of nitrogen-molecule reaction called S-nitrosylation can affect an important cellular protein called p62, triggering the build-up and spread of alpha-synuclein aggregates.
The p62 protein normally assists in autophagy, a waste-management system that helps cells get rid of potentially harmful protein aggregates. The researchers found evidence that in cell and animal models of Parkinson’s, p62 is S-nitrosylated at abnormally high levels in affected neurons. This alteration of p62 inhibits autophagy, causing a build-up of alpha-synuclein aggregates. The build-up of aggregates, in turn, leads to the secretion of the aggregates by affected neurons and some of these aggregates are taken up by nearby neurons.
The researchers also tested post-mortem brains of LBD patients and again found that levels of S-nitrosylated p62 were abnormally high in affected brain areas, supporting the idea that this process occurs in humans.
The researchers found that S-nitrosylation of proteins becomes more likely in many situations of cellular stress, including the presence of protein aggregates. Thus, this chemical modification of p62 could be a key factor in a self-reinforcing process that not only stresses brain cells beyond their limits, but also spreads the source of stress to other brain cells.
The team is now working to develop drug-like compounds that specifically inhibit the S-nitrosylation of p62. Although it would take years to develop such compounds as potential commercial drugs, they could, in principle, slow the Parkinson’s/LBD disease process or prevent its further spread in the brain after it begins.
Related topics
Disease Research, Drug Delivery, Drug Development, Drug Discovery, Drug Discovery Processes, Drug Leads, Neurons, Neurosciences, Protein
Related conditions
Lewy body dementia, Parkinson's disease
Related organisations
Scripps Research