Study suggests postnatal gene therapy could treat Pitt-Hopkins syndrome
Posted: 12 May 2022 | Ria Kakkad (Drug Target Review) | No comments yet
Researchers have found that restoring lost gene activity prevents many disease signs in an animal model of Pitt-Hopkins syndrome.


Scientists from The University of North Carolina (UNC) School of Medicine, US have shown for the first time that postnatal gene therapy may be able to prevent or reverse many deleterious effects of a rare genetic disorder called Pitt-Hopkins syndrome. The breakthrough was recently published in eLife.
The scientists developed an experimental, gene-therapy-like technique to restore the normal activity of the gene deficient in people with Pitt-Hopkins syndrome. In newborn mice that model the syndrome, the treatment prevented the emergence of disease signs including anxiety-like behaviour, memory problems, and abnormal gene expression patterns in affected brain cells.
Pitt-Hopkins syndrome arises in a child when one copy of the gene TCF4 is missing or mutated, resulting in an insufficient level of TCF4 protein. Typically, this deletion or mutation occurs spontaneously in the parental egg or sperm cell prior to conception, or in the earliest stages of embryonic life following conception.
Since TCF4 is a “transcription factor” gene, a master switch that controls the activities of at least hundreds of other genes, its disruption from the start of development leads to numerous developmental abnormalities. In principle, preventing those abnormalities by restoring normal TCF4 expression as early as possible is the best treatment strategy – but it is yet to be tested.
The researchers developed a mouse model of Pitt-Hopkins syndrome in which the level of the mouse version of TCF4 could be reliably halved. This mouse model showed many typical signs of the disorder. Restoring full activity of the gene from the start of embryonic life fully prevented these signs. The researchers also found evidence in these initial experiments that gene activity needed to be restored in essentially all types of neurons to prevent the emergence of Pitt-Hopkins signs.
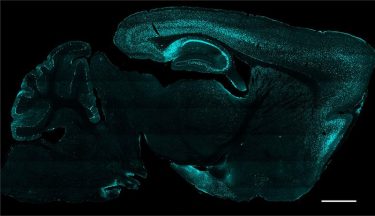

Brain section image: protein Cre (green) delivered to cells as gene therapy via AAV
[Credit: Philpot Lab, UNC School of Medicine].
The researchers next set up a proof-of-concept experiment modelling a real-world gene therapy strategy. In engineered mice in which roughly half the expression of the mouse version of Tcf4 was switched off, the researchers used a virus-delivered enzyme to switch the missing expression back on again in neurons, just after the mice were born. Analyses of the brains showed this restoration of activity over the next several weeks.
Even though the treated mice had moderately smaller brains and bodies compared to normal mice, they did not develop many of the abnormal behaviours seen in untreated Pitt-Hopkins model mice. The exception was innate nest-building behaviour, in which the treated mice seemed abnormal at first, although their abilities were restored to normal within a few weeks.
The treatment at least partly reversed two other abnormalities seen in untreated mice: altered levels of the genes regulated by TCF4 and altered patterns of neuronal activity as measured in electroencephalograph (EEG) recordings.
The researchers now plan to explore the effectiveness of their strategy when applied to Pitt-Hopkins mice at later stages of life. They also plan to develop an experimental gene therapy in which the human TCF4 gene itself will be delivered by a virus into a Pitt-Hopkins mouse model – a therapy that ultimately could be tested in children with Pitt-Hopkins syndrome.
Related topics
Disease Research, Gene Testing, Gene Therapy, Therapeutics
Related conditions
Pitt-Hopkins Syndrome
Related organisations
University of North Carolina (UNC) School of Medicine