New study demonstrates an in silico reaction screening strategy for reaction development
Posted: 23 September 2022 | Ria Kakkad (Drug Target Review) | No comments yet
Through the novel screening strategy, the researchers were able to develop a suit of 48 reactions that produce compounds that are potentially useful for new drugs.


In a new study, researchers at Institute for Chemical Reaction Design and Discovery (ICReDD), Japan, used computer stimulations to successfully develop a suite of 48 reactions that produce compounds potentially useful for novel drug development. Details of the new screening strategy were recently published in Nature Synthesis.
The presence and position of fluorine in a molecule often affects a molecule’s pharmacological activity. The team used quantum chemical calculations to discover a reaction that selectively adds two fluorine atoms to a difficult-to-access position on an N-heterocycle — molecules with a carbon ring structure where at least one carbon in the ring is replaced with nitrogen. The ability to attach fluorine atoms to the previously difficult-to-access “alpha carbon” — the carbon immediately next to the nitrogen in the ring structure — could lead to the development of a host of novel drugs.
Before carrying out experiments in the lab, the researchers cast a wide net, computationally testing the viability of numerous 3-component reactions using the artificial force induced reaction (AFIR) method. They simulated the reaction of a difluorocarbene molecule, which acts at the source of fluorine atoms, with various pairs of small molecules featuring a double or triple bond. These simulations showed that several ring-forming reactions should be viable.
Researchers tried one of the promising reactions suggested by initial computational results but were not successful. A more narrowly focused, optimised computation of the transition state energy of the reaction in question showed that the difluorocarbene molecule more easily reacted with itself than with the desired starting molecules, signalling that an undesired side reaction was likely occurring. This result inspired researchers to change one of the starting materials to the cyclic molecule pyridine, which they anticipated would be able to compete with the unwanted side reaction. This change resulted in the successful synthesis of the desired N-heterocyclic product with two fluorines attached at the alpha carbon position.
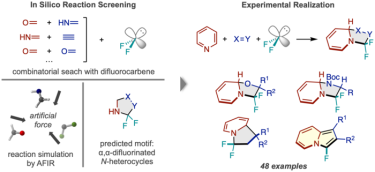

Workflow of reaction discovery via in-silico screening. (Left) Reactions between difluorocarbene and numerous pairs of small molecules were simulated, predicting a N-heterocycle product fluorinated twice at the alpha carbon. (Right) The successful reaction framework using pyridine and examples of the types of product compounds obtained.
[Credit: Hiroki Hayashi, Hitomi Katsuyama, Hideaki Takano, Yu Harabuchi, Satoshi Maeda, Tsuyoshi Mita. Nature Synthesis].
The reaction developed here is also significant because it breaks the aromatic system of electrons in the pyridine molecule, a transformation that is especially difficult due to the high stability of aromatic systems. Additionally, the 3-component reaction framework was applied successfully in the lab to a wide range of starting materials, resulting in many new molecules with unique alpha position fluorine substitutions. The large scope of reactivity greatly increases the potential utility of this reaction framework in new drug development.
The researchers see their streamlined screening method to broaden the scope of their search and discover new horizons in chemical reaction design.
“Our study’s highlight is the successful demonstration of an in silico reaction screening strategy for reaction development. The computational reaction simulation suggested less-explored three-component reactions of difluorocarbene and two unsaturated molecules, which we successfully realised in experiments,” explained lead author Hiroki Hayashi. “I think the AFIR method is a powerful tool for dictating new research directions in reaction discovery, and we plan to continue building a computation-based reaction development platform by integrating the computational and informatics techniques of ICReDD.”
Missed the news? Researchers have developed a new method that enables high-throughput screening of Huntington’s disease organoids.
READ MORE
Related topics
Screening
Related organisations
Institute for Chemical Reaction Design and Discovery (ICReDD)
Related people
Hiroki Hayashi