Lipid nanoparticle delivery of mRNA could lead to new ‘universal’ COVID-19 treatment
Posted: 19 October 2022 | Victoria Rees (Drug Target Review) | No comments yet
A study has shown that mRNA delivered via lipid nanoparticles blocks multiple variants of SARS-CoV-2 from entering cells in mice.


A study at Oregon State University (OSU), US has demonstrated a proof-of-principle for a new “universal” means of treating COVID-19. The researchers showed in a mouse model that it is possible to use messenger RNA (mRNA) to prompt the production of a protein that can block multiple variants of the SARS-CoV-2 virus from entering cells and causing respiratory disease.
“Rather than mRNA as a vaccine, this shows that mRNA can be used as a universal therapy against different coronaviruses,” said Associate Professor Gaurav Sahay, lead researcher from the study. “Despite mass vaccination, there is an urgent need to develop effective treatment options to end this pandemic. Several therapies have shown some effectiveness, but the virus’ high mutation rate complicates the development of drugs that treat all variants of concern.”
The findings, published in Advanced Science, show that delivering mRNA packaged in lipid nanoparticles to mice can cause host cells to produce a “decoy” enzyme that binds to coronavirus spike proteins, meaning the virus should not be able to latch onto cells in the host’s airway and start the infection process.
“Proteins are large, complex molecules that serve as the workhorses of cells, enabling all of the biological functions within a cell,” said Sahay. “DNA holds the blueprints from which proteins get made after the code is first transcribed into mRNA.”
Human angiotensin-converting enzyme 2 (hACE2) is an enzyme of the airway cells. It is also expressed in the heart, kidney and intestine and is involved in numerous physiological functions. The researchers say that simply giving a COVID-19 patient hACE2 would have limited effectiveness in treating the disease because the soluble form of the enzyme, the kind that can circulate throughout the body, has a short half-life – less than two hours. However, lipid nanoparticles containing mRNA that order production of the enzyme can help overcome that problem.
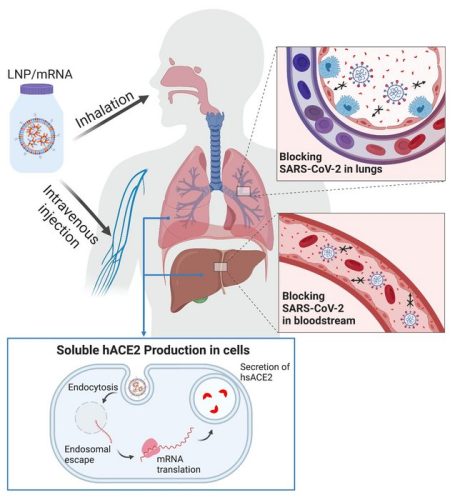

Depiction of mRNA therapy [credit: OSU College of Pharmacy].
In this study, the researchers engineered synthetic mRNA to encode a soluble form of the enzyme, packaged the mRNA into lipid nanoparticles and delivered it to cells in the liver intravenously; within two hours, the enzyme was in the mice’s bloodstream and stayed there for days. The scientists also delivered the loaded lipid nanoparticles via inhalation, prompting epithelial cells in the lungs to secrete soluble hACE2.
“The soluble enzyme effectively inhibited live SARS-CoV-2 from infecting host cells,” said OSU postdoctoral researcher Jeonghwan Kim. “The synthesis of mRNA is fast, affordable and scalable and lipid nanoparticle-delivered mRNA can be repeated as necessary to sustain protein production until the infection subsides. Once treatment stops, the no-longer-needed soluble hACE2 clears the system in a matter of days.”
Related topics
Drug Delivery, In Vivo, Lipidomics, Lipids, Nanomedicine, Nanoparticles, RNAs, Therapeutics
Related conditions
Covid-19
Related organisations
Oregon State University
Related people
Associate Professor Gaurav Sahay, Jeonghwan Kim