Trioxacarcins synthesised as a potent anti-cancer agent
Posted: 11 March 2016 | Victoria White | No comments yet
Trioxacarcins disrupt the replication of cancer cells by binding and chemically modifying their genetic material…
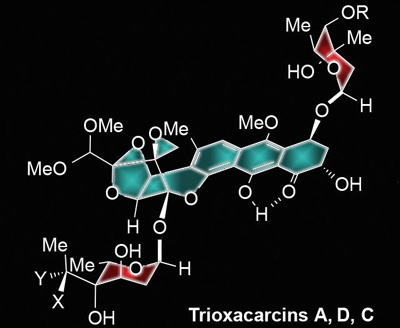

A schematic shows a trioxacarcin C molecule, whose structure was revealed for the first time through a new process developed by K.C. Nicolaou’s lab. CREDIT: Nicolaou Group/Rice University
A team led by Rice University’s K.C. Nicolaou has developed a new process for the synthesis of a series of potent anti-cancer agents originally found in bacteria.
The Nicolaou lab finds ways to replicate rare, naturally occurring compounds in larger amounts so they can be studied by biologists and clinicians as potential new medications. It also seeks to fine-tune the molecular structures of these compounds through analogue design and synthesis to improve their disease-fighting properties and lessen their side effects. Such is the case with their synthesis of trioxacarcins.
“Not only does this synthesis render these valuable molecules readily available for biological investigation, but it also allows the previously unknown full structural elucidation of one of them,” Nicolaou said. “The newly developed synthetic technologies will allow us to construct variations for biological evaluation as part of a program to optimise their pharmacological profiles.”
At present, there are no drugs based on trioxacarcins, which damage DNA through a novel mechanism, Nicolaou said.
Trioxacarcins bind and chemically modify cancer cell genetic material
Trioxacarcins were discovered in the fermentation broth of the bacterial strain Streptomyces bottropensis. They disrupt the replication of cancer cells by binding and chemically modifying their genetic material.
“These molecules are endowed with powerful anti-tumour properties,” Nicolaou said. “They are not as potent as shishijimicin, which we also synthesised recently, but they are more powerful than taxol, the widely used anti-cancer drug. Our objective is to make it more powerful through fine-tuning its structure.”
Nicolaou said his lab is working with a biotechnology partner to pair these cytotoxic compounds (called payloads) to cancer cell-targeting antibodies through chemical linkers. The process produces antibody-drug conjugates as drugs to treat cancer patients.
“It’s one of the latest frontiers in personalised targeting chemotherapies,” said Nicolaou, who earlier this year won the prestigious Wolf Prize in Chemistry.
Related topics
Oncology
Related organisations
Rice University