New treatment pathway for deadly pancreatic cancers
Posted: 25 April 2023 | Izzy Wood (Drug Target Review) | No comments yet
US scientists have used mouse models of pancreatic cancer to identify genes used by tumour cells to grow uncontrollably.

Researchers at the Johns Hopkins Kimmel Cancer Centre, US, have identified a novel cell signalling pathway that potentially could be targeted in therapeutics for aggressive pancreatic cancers.
In laboratory studies with human pancreatic cancer cell lines and genetically engineered mouse models of pancreatic cancer, the investigators discovered that the High Mobility Group A1 (HMGA1) protein functions as a “molecular switch” that “flips on” genes required by tumour cells to grow in an uncontrolled fashion and form invasive tumours. The study was recently published in The Journal of Clinical Investigation.
One of these genes activated by HMGA1 leads to the production of fibroblast growth factor 19 (FGF19), which is secreted by tumour cells. FGF19 not only provides signals that coax tumour cells to grow rapidly and invade surrounding tissues, but both HMGA1 and FGF19 cooperate to “build” a dense, fibrous, scar-like wall around the tumour cells, which is known as the stroma.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
When the scientists silenced HMGA1 or disrupted FGF19 signals in mouse models of pancreatic cancer, tumour cells had markedly decreased growth and less stroma formation, suggesting that drugs to block FGF19 signals already available for use by patients with other diseases could be repurposed to treat pancreatic tumours that have high levels of FGF19. Studies of cancer genomes indicate that up to a quarter of human pancreatic cancers have high levels of HMGA1 and FGF19.
“In prior work, we found that HMGA1 was overexpressed in most pancreatic cancers and very late stage precursor lesions, as well as in other aggressive tumours such as leukaemia and advanced stage myeloproliferative neoplasms, which suggested to us that HMGA1 was playing a fundamental role in driving tumours progression,” said study author Dr Linda Resar, Professor of Medicine, Oncology and Pathology at Johns Hopkins
In a series of laboratory experiments, the researchers investigated several methods of disrupting HMGA1 and FGF19. First, they silenced HMGA1 in pancreatic cancer cell lines from primary and metastatic tumours using short hairpin RNA—or artificial RNA molecules that block gene expression. It was observed that a deficiency in HMGA1 led to decreased growth rates, impairing migration, invasion and other cancerous properties. They also developed mouse models of pancreatic cancer missing one or two mouse genes for HMGA1 in the pancreas. Surprisingly, loss of just one gene was sufficient to slow tumours formation and progression.
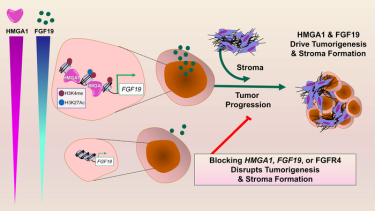

Pancreatic tumors with high expression of HMGA1 and FGF19 comprise a subset of human pancreatic cancers with extremely poor outcomes. Most importantly, inhibitors to FGF19 receptors (FGFR4) are already available in the clinics for other diseases and provide a potential new therapy for these highly lethal pancreatic tumors.
In additional tests, researchers found that FGF19 gene expression, protein levels in pancreatic cancer cells and secretion all depend on HMGA1. Also, silencing FGF19 mirrored effects of silencing HMGA1, decreasing tumour growth and invasive properties. Most importantly, administration of BLU9931, a small-molecule drug that inhibits FGFR4 (a receptor for FGF19), led to decreased tumour growth and stroma formation in mouse tumours.
“Together, we discovered what we believe is a previously undescribed paradigm whereby tumour cells collaborate via HMGA1 and FGF19 to drive cancer progression and stroma formation,” Resar added. “This work also unveiled FGF19 as a potential therapeutic target for a very aggressive subset of human pancreatic cancers. Perhaps the most exciting aspect of our studies is that inhibitors to FGF19 are available and have already been tested in humans.”
In ongoing studies, the researchers are evaluating additional tumours to determine if they upregulate FGF19 and could benefit from treatment with FGF19 blockade. The researchers will also test whether inhibiting FGF19 blocks the spread or metastasis of tumour cells to distant sites.
Related topics
Animal Models, Genetic Analysis, Genome Editing, Genomics, Oncology, Protein, Protein Expression, Targets, Therapeutics
Related conditions
Pancreatic cancer
Related organisations
Johns Hopkins Kimmel Cancer Centre
Related people
Dr Linda Resar