Alcohol-associated liver disease linked to abnormal splicing deregulation
Posted: 27 July 2023 | Izzy Wood (Drug Target Review) | No comments yet
Splicing deregulation is potential drug target and diagnostic tool, but can be affected by alcohol consumption and thus chronic liver disease.
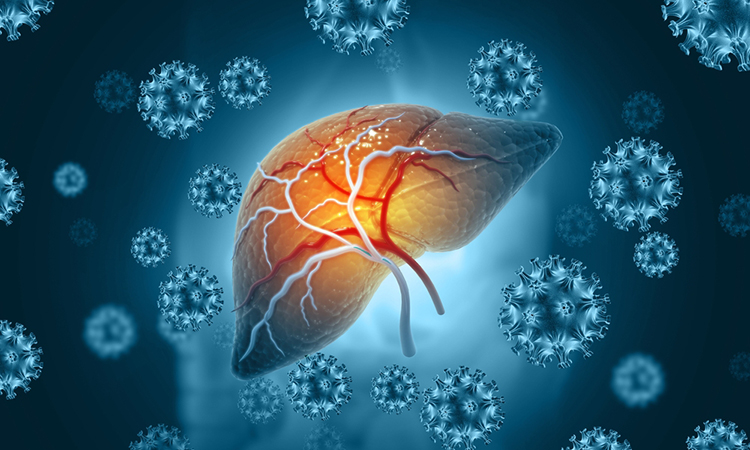

Alcohol consumption is a major cause of chronic liver disease. The disease, which has devastating consequences, progresses through stages of hepatitis (inflammation), fibrosis (scarring) and cirrhosis (severe scarring). Advanced disease increases the risk of developing liver cancer, and some individuals may require a liver transplant to live.
Despite a tremendous need for new treatments, the specific biological factors that determine when and how rapidly liver cells deteriorate remain largely unknown.
In a study published in Hepatology, a research team at The University of Texas Health Science Centre at San Antonio (UT Health San Antonio), US, shines light on the disease process, including a possible therapeutic approach for combating alcohol-associated liver disease.
The study’s senior author, who is Professor of Molecular Medicine within the health science centre’s Sam and Ann Barshop Institute for Longevity and Aging Studies, Dr Mengwei Zang explained: “In our study, we show that the cutting and rejoining of molecules called pre-messenger RNAs is associated with liver disease caused by excessive alcohol consumption.”
“We observed this link in both mice and humans, shedding light on the biological role of pre-mRNA splicing in maintaining steady liver metabolic function,” Zang continued . “Moreover, our findings suggest that targeting specific splicing factors in liver cells called hepatocytes could offer a novel approach to mitigate alcohol-associated liver disease.”
It’s been known that certain splicing defects can lead to cancer cell processes, but the UT Health San Antonio team was intrigued about the role of abnormal pre-mRNA splicing in driving alcohol-related liver disease since practically nothing is known about the latter, Zang said.
One of Zang lab’s findings hints that splicing activity could help predict liver fat accumulation in individuals even before advanced disease such as fibrosis is apparent.
“This is a highly interesting result and suggests that splicing deregulation could potentially be used as an early indicator of fatty liver disease,” Zang added.
In mouse studies, the UT Health San Antonio team studied FGF21, a protein secreted by liver cells. FGF21 eased the alcohol-associated liver disease, possibly by inhibiting a splicing protein called SRPK2.
“Targeting SRPK2 signalling by FGF21 may represent a potential therapeutic approach for combating alcohol-associated liver disease,” Zang concluded.
Related topics
Protein, Protein Expression, RNAs
Related conditions
chronic liver disease, cirrhosis (severe scarring), fibrosis (scarring), hepatitis (inflammation)
Related organisations
The University of Texas Health Science Centre at San Antonio (UT Health San Antonio)
Related people
Dr Mengwei Zang