New understanding of early breast cancer development
Posted: 2 November 2023 | Drug Target Review | No comments yet
Mammary organoid cultures show the effects of BRCA2 mutations on breast tissue cells, increasing the likelihood of early, targeted treatment.
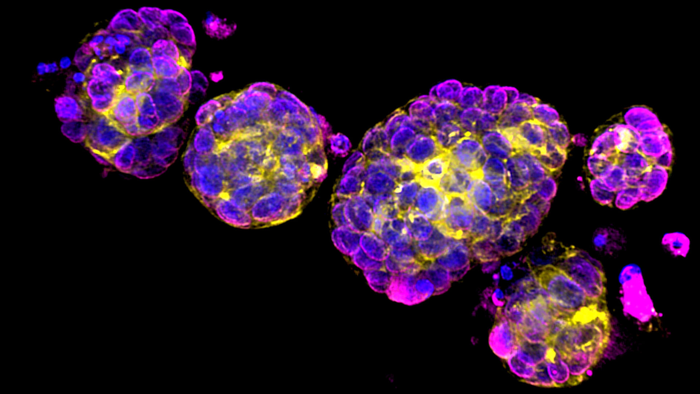

Organoids are tiny clumps of cells that are grown from and resemble tiny 3D organs. The image above shows breast cancer organoids, derived from human patients. Breast cancer cells are labeled in purple, DNA is labeled in blue, and cytoskeleton proteins are labeled yellow. Organoids provide a more natural setting than flat tissue culture dishes to study how cancer cells grow, develop, and can be treated [Credit: Sonam Bhatia/Spector lab/CSHL, 2022}.
Researchers have made crucial insights into the effects of BRCA2 mutations on breast tissue cells which explain early breast cancer development in people with BRCA2 mutations. The study was led by Professor Ashok Venkitaraman, Director of the Cancer Science Institute of Singapore (CSI Singapore) at the National University of Singapore and Dr Mona Shehata from the University of Cambridge (UK).
For people with BRCA2 mutations breast cancer is a serious concern, with approximately 70 percent of carriers developing the disease by 80 years of age. Although BRCA2’s role in DNA repair and as a tumour suppressor in protecting the cells against DNA replication stress are well known, there is lots to be discovered about the pathways leading to breast cancer development in BRCA2 mutation carriers.
Dr Venkitaraman said: “Despite the high risk faced by BRCA2 mutation carriers in developing breast cancer, there is still a critical lack of understanding of how these mutations impact the various cell types in the mammary glands. Our study aims to unravel the earliest event preceding the formation of cancer within the breast tissue microenvironment to better design early intervention strategies.”
An investigation using long-term mammary organoid cultures showed that healthy mammary glands are not predisposed to form lesions that precede tumours. However, if, during DNA replication, they are exposed to stress, the population of hormone receptor-negative (HR-) luminal cells significantly expanded. The expansion was particularly robust among the mammary gland organoids with BRCA2 mutations.
The team also discovered that HR- luminal cells with BRCA2 mutation demonstrated enhanced organoid formation and survival under replication stress. Further analysis at the single-cell RNA-sequencing level found elevated stemness markers and Type I interferon responses in these cells, which favours the growth of HR- luminal cells. HR- luminal BRCA2-mutant cells also became more susceptible to tumour formation only after multiple stresses, suggesting that distinct mechanisms direct the transformation of HR- and HR+ tumours, specifically in BRCA2 mutation carriers.
It is vital to understand the mechanisms that drive early cancer development in order to classify breast cancers into their distinct subtypes, like triple-negative breast cancers, and hormone positive and HER2 positive breast cancers, so that targeted, early treatment can be given to achieve positive outcomes.
The researchers aim to further validate this study’s findings in mammary glands from preventive mastectomy cases among BRCA2 carriers. This will improve the evidence to design early intervention strategies, providing guidance to clinicians caring for individuals with BRCA2 mutations.
This study was published in Nature Communications.
Related conditions
Breast cancer
Related organisations
Cancer Science Institute of Singapore (CSI Singapore), University of Cambridge