How pathological alpha synuclein causes neuron death
Posted: 20 December 2023 | Drug Target Review | No comments yet
A new finding that pathological alpha-synuclein causes cells to increase protein synthesis suggests new targets for treating PD.
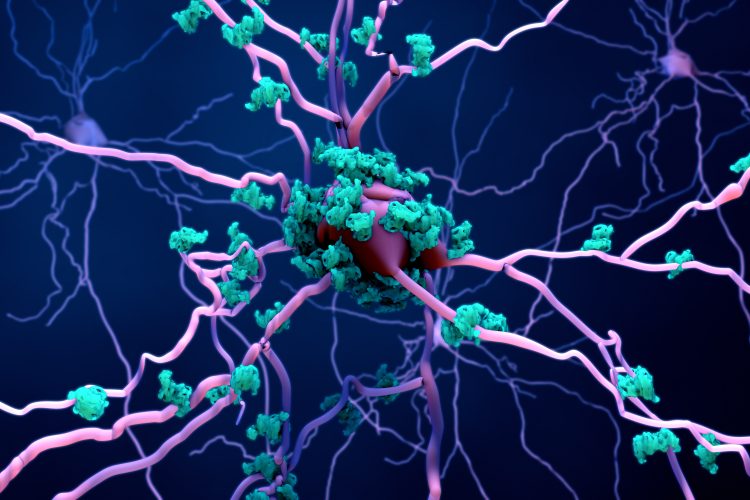

Scientists from the Johns Hopkins University School of Medicine have discovered that a pathological protein associated with Parkinson’s disease (PD) triggers cells to increase protein synthesis, which eventually kills the subset of brain cells that die off in this condition. The study’s findings offer potential new targets for treating PD.
Dr Ted Dawson, study leader, Professor in the Department of Neurology and director of the Institute for Cell Engineering at the Johns Hopkins University School of Medicine said: “We hope that research like this will provide mechanistic, molecular-based therapies that can actually slow or halt the progression of Parkinson’s disease.”
PD symptoms include several motor and cognitive deficits that worsen over time. These result from the death of neurons that produce the chemical messenger dopamine. Current treatments with drugs such as L-dopa largely focus on replacing the dopamine lost when these dopaminergic neurons die.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
Scientists have linked these cells’ death to the presence of a pathological form of alpha-synuclein, a normal protein that is abundant in brain tissue, although how it causes dopaminergic neuron death was unknown. To determine its role, Dawson and his colleagues used proximity labelling with mass spectrometry to identify proteins that might interact with pathological alpha-synuclein in both a mouse and a lab cell model of Parkinson’s neurons.
The team found 100 such proteins that overlapped between these two models. They found the majority play roles in ribonucleic acid (RNA) processing and translation initiation, key processes used by cells to make new proteins, when the researchers grouped the proteins by function. Several of the proteins were already known to work with the mammalian target of rapamycin (mTOR), which both regulates protein production and breaks down proteins.
Mice were genetically manipulated to over-express the pathological form of alpha-synuclein. The experiments using these mice showed that pathological alpha-synuclein indeed caused cells to increase protein synthesis by activating mTOR.
This process was initiated when the pathological alpha-synuclein bound to another protein, tuberous sclerosis complex 2 (TSC2). This prevented it from connecting with yet another protein TSC1, that keeps mTOR in check.
When the genetically engineered mice were treated with rapamycin, a drug that targets mTOR, it prevented excessive protein production in mice with a condition like Parkinson’s disease, and eased some of the slow, halting movements and weak grip strength that are hallmarks of Parkinson’s disease in patients.
Dawson noted that it remains unclear how increased protein production might harm dopaminergic neurons. The proteins might clog up key cellular pathways, or specific proteins produced in excess might be harmful for cells. This will be investigated in future research.
In the meantime, Dawson said the results suggest new targets for treating Parkinson’s disease. Researchers may, for example, develop drugs that act like rapamycin, currently used as an anti-rejection and anti-cancer medication, but work specifically in the brain to save dopaminergic neurons, sparing patients unnecessary body-wide side effects.
The study was published in Science Translational Medicine.
Related topics
Neurons, Neurosciences, Protein
Related conditions
Parkinson's disease
Related organisations
Johns Hopkins University School of Medicine.