Model for spinal muscular atrophy using human iPSCs
Posted: 21 December 2023 | Drug Target Review | No comments yet
A 2D neuromuscular junction model enables high-throughput screening to discover new treatments for neuromuscular diseases.
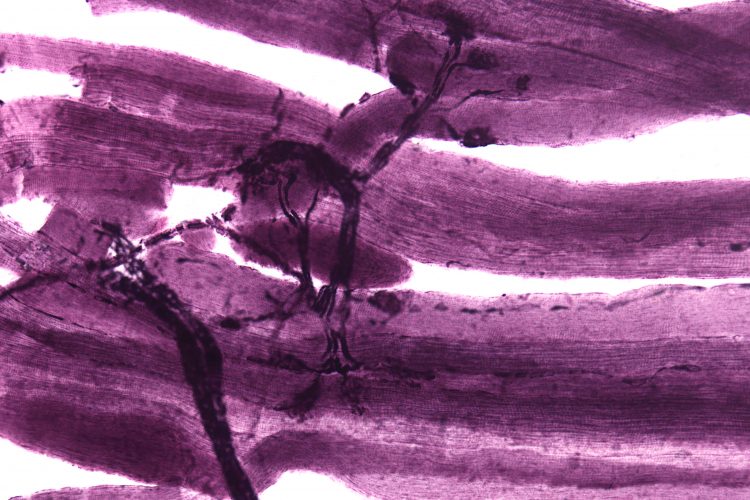

A microscopy image of neuromuscular junctions with projections from the neurons (grey/black) connecting to the muscles (purple).
Researchers at the Max Delbrück Center for Molecular Medicine at the Helmholtz Association have developed a neuromuscular model for drug development. So far, around 800 different neuromuscular diseases have been found, and cause issues in the way muscle cells, motor neurons and peripheral cells interact. These disorders, including amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA), lead to muscle weakness, paralysis, and in some cases death.
Dr Mina Gouti, head of the Stem Cell Modeling of Development and Disease Lab at the Max Delbrück Center said: “These diseases are highly complex, and the causes of the dysfunction can vary widely.” The problem could be with the neurons, the muscle cells, or the connections between the two. “To better understand the causes and find effective therapies, we need human-specific cell culture models where we can study how motor neurons in the spinal cord interact with the muscle cells.”
Two-dimensional model
A three-dimensional (3D) neuromuscular organoid (NMO) system had already been developed by the researchers working with Gouti. However, Gouti stated: “One of our goals is to use our cultures for large-scale drug testing…The three-dimensional organoids are very large and can’t be grown for a long time in the 96 well culture dish that we use to perform high-throughput drug screening studies.”
For high-throughput screening, the team developed a self-organising neuromuscular junction model using pluripotent stem cells, containing neurons, muscle cells, and the chemical synapse named neuromuscular junction that is needed for the two types of cells to interact. “The 2D self-organising neuromuscular junction model will allow us to perform high throughput drug screening for different neuromuscular diseases and then study the most promising candidates in patient-specific organoids,” explained Gouti.
First, the researchers had to understand how motor neurons and muscle cells develop in the embryo to establish the 2D model. Gouti’s team does not conduct embryonic research themselves but utilises various human stem cell lines, which are allowed for research purposes under strict guidelines, and an induced pluripotent stem cell line (iPSC).
Lead author of the paper Alessia Urzi, a doctoral student, said: “We tested a number of hypotheses. We found that the types of cells we needed for functional neuromuscular connections originated from neuromesodermal progenitor cells.” Urzi identified the correct combination of signalling molecules that cause human stem cells to mature into functional motor neurons and muscle cells with the necessary connections between the two. The team also observed that, once differentiated, the cells organised themselves into areas with muscle cells and nerve cells.
Activating motor neurons
Although the muscle cells grown in the culture dish contract spontaneously because of their connection to the neurons, they do so without any meaningful rhythm. To fix this, Urzi and Gouti worked with researchers at Charité – Universitätsmedizin Berlin. Using optogenetics, they activated the motor neurons. Once activated by a flash of light, the neurons cause the muscle cells to contract in sync, moving them closer to mimicking the physiological situation in an organism.
Spinal muscular atrophy
Urzi used human iPSCs from patients with spinal muscular atrophy, a severe neuromuscular disease that affects children in the first year of their life, to test the validity of the model. The neuromuscular cultures generated from the patient-specific induced pluripotent stem cells showed serious problems with the contraction of the muscle resembling the patient’s pathology.
For Gouti, the 2D and 3D cultures are essential tools for researching neuromuscular diseases in better detail and to test more efficient and individualised treatment options. Next, the team aim to perform high throughput drug screening to find novel treatments for patients with spinal muscular atrophy and amyotrophic lateral sclerosis.
Gouti concluded: “We want to start by seeing if we can achieve more successful outcomes using new combinations of drugs to improve the life of patients with complex neuromuscular diseases.”
The study is published in Nature Communications.
Related topics
High-Throughput Screening (HTS), Neurons, Organoids, Stem Cells
Related conditions
Amyotrophic Lateral Sclerosis (ALS), neuromuscular disorders, spinal muscular atrophy (SMA)
Related organisations
Charité Universitätsmedizin Berlin, Max Delbrück Center for Molecular Medicine