Antibiotics weaken Alzheimer’s disease progression
Posted: 21 July 2016 | Niamh Louise Marriott, Digital Content Producer | 1 comment
Antibiotic treatment decreases levels of disease-causing plaques, a hallmark of Alzheimer’s disease, according to a new study by neuroscientists…


Long term antibiotic treatment decreases levels of disease-causing plaques, a hallmark of Alzheimer’s disease, and enhances neuroinflammatory activity of microglial cells, according to a new study by neuroscientists from the University of Chicago.
The study, using mice, showed significant changes in the gut microbiome after antibiotic treatment, suggesting the composition and diversity of bacteria in the gut play an important role in regulating immune system activity that impacts progression of Alzheimer’s disease.
Alzheimer’s disease, the most common type of dementia, is a progressive neurological disease which affects multiple brain functions, including memory and affects an estimated 850,000 people in the UK.
“We’re exploring very new territory in how the gut influences brain health,” said Sangram Sisodia, PhD Professor of Neurosciences at the University of Chicago. “This is an area that people who work with neurodegenerative diseases are going to be increasingly interested in, because it could have an influence down the road on treatments.”
Attacking Alzheimer’s from the core
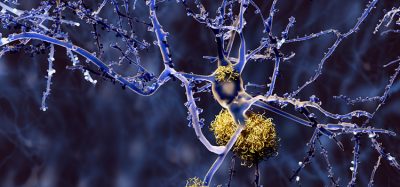
Two of the key features of Alzheimer’s disease are the development of amyloidosis, accumulation of amyloid-ß (Aß) peptides in the brain, and inflammation of the microglia, brain cells that perform immune system functions in the central nervous system. Buildup of Aß into plaques plays a central role in the onset of Alzheimer’s, while the severity of neuro-inflammation is believed to influence the rate of cognitive decline from the disease.
For this study, Sisodia and his team administered high doses of broad-spectrum antibiotics to mice over five to six months. At the end of this period, genetic analysis of gut bacteria from the antibiotic-treated mice showed that while the total mass of microbes present was roughly the same as in controls, the diversity of the community changed dramatically.
A huge decrease in amyloid-ß peptides
The antibiotic-treated mice also showed more than a two-fold decrease in Aß plaques compared to controls, and a significant elevation in the inflammatory state of microglia in the brain. Levels of important signalling chemicals circulating in the blood were also elevated in the treated mice.
While the mechanisms linking these changes is unclear, the study points to the potential in further research on the gut microbiome’s influence on the brain and nervous system.
A viable treatment?
“We don’t propose that a long-term course of antibiotics is going to be a treatment–that’s just absurd for a whole number of reasons,” said Myles Minter, PhD, a postdoctoral scholar in the Department of Neurobiology, University of Chicago. “But what this study does is allow us to explore further, now that we’re clearly changing the gut microbial population and have new bugs that are more prevalent in mice with altered amyloid deposition after antibiotics.”
Minter said the collaboration was enabling, and highlighted the cross-disciplinary thinking necessary to tackle a seemingly intractable disease like Alzheimer’s. “Once you put ideas together from different fields that have largely long been believed to be segregated from one another, the possibilities are really amazing,” he said.
Moving towards a cure
Sisodia cautioned that while the current study opens new possibilities for understanding the role of the gut microbiome in Alzheimer’s disease, it’s just a beginning step.
“There’s probably not going to be a cure for Alzheimer’s disease for several generations, because we know there are changes occurring in the brain and central nervous system 15 to 20 years before clinical onset,” he said. “We have to find ways to intervene when a patient starts showing clinical signs, and if we learn how changes in gut bacteria affect onset or progression, or how the molecules they produce interact with the nervous system, we could use that to create a new kind of personalized medicine.”
Related topics
Antibiotics, Genetic Analysis, Microbiome, microglial cells, Neurosciences
Related conditions
Alzheimer’s disease, amyloidosis, Neurodegenerative diseases, Neuroinflammatory activity







The Gut Microbial Endocrine Organ: Bacterially-Derived Signals Driving Cardiometabolic Diseases
J. Mark Brown, Ph.D. and Stanley L. Hazen, M.D., Ph.D.
Department of Cellular and Molecular Medicine, Cleveland Clinic Lerner Research Institute, Cleveland, OH 44195, USA
Annu Rev Med 2015 ; 66: 343–359. doi:10.1146/annurev-med-060513-093205.