The emergence of targeted alpha therapy for glioblastoma
Posted: 16 July 2024 | Drug Target Review | No comments yet
Targeted alpha therapy shows promising preclinical results, increasing survival rate by 36.4 percent in recurrent tumours.
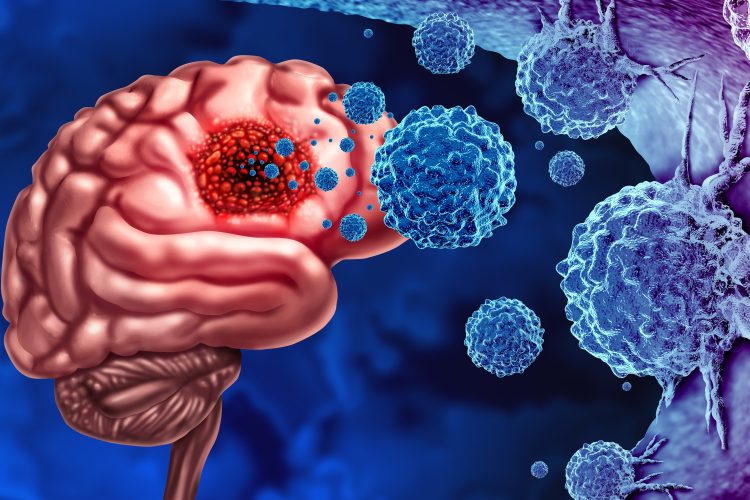

Researchers at the University of South Australia (UniSA) have considered a novel approach of treating glioblastoma, which has demonstrated promising results in preclinical settings. This could increase average survival rates beyond the current 18 months.
Glioblastoma (GB) is the most malignant type of brain cancer, with a strong resistance to existing therapies. At present, the standard treatment for GB is surgery, external beam radiotherapy and the chemotherapy drug temozolomide. However, as the survival rate is less than five to 10 percent at five years, researchers are investigating alternatives, in which targeted alpha therapy (TAT) is emerging as a potential additional treatment.
Dr Frank Saran, an Adjunct Clinical Professor at UniSA and radiation oncologist, spoke of the limited progress. “Research into this area is very low for several reasons. First, glioblastoma is a rare cancer so does not affect large swathes of the population. It also has extremely low survival rates and there is a long-standing history of failed studies in this area. Unfortunately, pharmaceutical companies are often not willing to invest money in GB because it has a low probability of success and is not commercially viable.”
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
In the new study, the team conducted their own experiments with TAT, reviewing existing clinical studies to evaluate the viability of TAT as a treatment option for recurrent glioblastoma. Maram El Sabri, PhD candidate at UniSA commented: “Unlike external beam radiotherapy, which delivers radiation more diffusely over a larger volume, TAT delivers high amounts of lethal radiation to the tumour at very short range, hitting its target without significantly affecting surrounding healthy tissue.”
It is challenging for oncologists to determine the optimal dosage of radiation required to destroy GB, as the tumours grow rapidly and metastasise beyond the visible tumour into normal brain tissue. Animal studies show that only a few targeting agents can effectively cross the blood brain barrier (BBB) to reach cancerous tissue. Those that do cause undesirable side effects in surrounding healthy tissue.
TAT has demonstrated an increase survival rates by 16.1 percent in newly diagnosed GB cases and by 36.4 percent in recurrent tumours in the preclinical experiments. These studies also suggest that it has minimal adverse effects for the patient.
Dr Eva Bezak, co-author and medical radiation physicist, commented that TAT was first proposed for cancer therapy over 20 years ago by internationally acclaimed Australian research scientist, Professor Barry Allen. “He was ahead of his time. It has taken this long for TAT to be gradually accepted by clinicians and for animal (pre-clinical) and human (clinical) studies to be undertaken.”
She added. “Pre-clinical studies show very promising results. Alpha emitters are up to 10 times more toxic to cells than gamma radiation, which are used in external beam radiation. Also, compared to the cost of current immunotherapy or molecular targeting drugs, targeted alpha therapy is relatively cheap.”
Maram is developing a computational model in her PhD to calculate how TAT can be delivered most effectively to the brain after surgery, as well as in combination with radiation treatment and chemotherapies. She concluded: “I am excited to find out if we can find the right dosing and radiation range by adding TAT to the conventional treatment options. If this is successful, we might see some significant results in terms of extending a patient’s life.”
This study was published in Targeted Oncology.
Related topics
Chemotherapy, Disease Research, Oncology, Radiotherapy
Related conditions
Glioblastoma
Related organisations
University of South Australia