TLE6 protein deficiency linked to male infertility
Posted: 27 January 2025 | Drug Target Review | No comments yet
New study reveals that TLE6 protein deficiency causes male infertility in mice. These findings suggest potential genetic causes and future treatment avenues for male infertility.
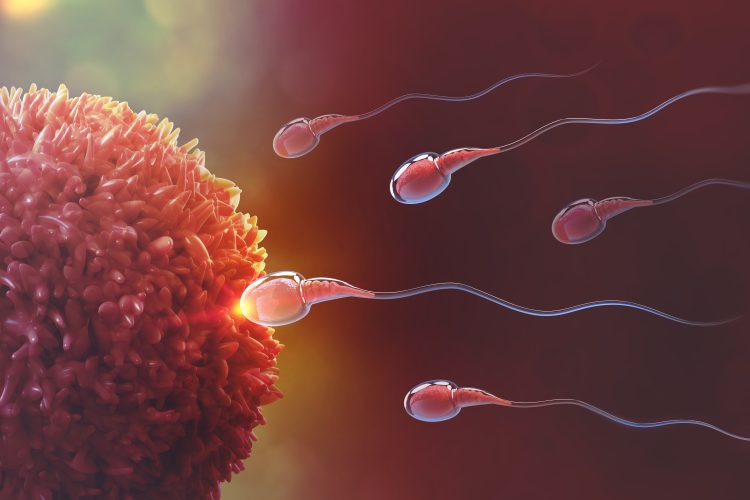

Genetic changes are a significant cause of infertility, affecting over 15 percent of the global population. TLE6, a protein critical in early embryonic development, has long been known to influence female fertility. However, new research reveals its unexpected role in male fertility, linking a deficiency in TLE6 to infertility in male mice. A new study provides valuable insights into the genetic underpinnings of male infertility, shedding light on potential avenues for treatment and further research.
Genetic mutations affecting early embryonic development, egg maturation, and fertilisation have become key areas of focus in understanding infertility. One of the most well-studied causes of early embryonic infertility involves mutations in genes related to the subcortical maternal complex (SCMC). SCMC plays a crucial role in maintaining the integrity of the egg cytoplasm and facilitating proper embryo formation. The transducin-like enhancer of split 6 (TLE6) protein is a vital member of this complex, and its absence in females has been shown to disrupt embryo development, often leading to fragmentation and death after the two-cell stage. While TLE6’s role in female fertility is well established, its function in male germ cells remained largely unexplored until now.
A team of researchers from Kanazawa Medical University in Japan, led by Kousuke Kazama, sought to address this gap in understanding by investigating the impact of TLE6 deficiency on male fertility. Using a novel TLE6-deficient mouse model, they used CRISPR-Cas9 gene-editing technology to generate TLE6 hetero knockout male mice. Their findings, published in Frontiers in Cell and Developmental Biology, present compelling evidence that TLE6 plays a significant role in male fertility.
“We generated TLE6 hetero knockout mice to investigate the effects of TLE6 deficiency in male mice. We performed genome editing of the embryos using the CRISPR-Cas9 system and electroporation to generate the TLE6 hetero knockout mice,” says Kazama, explaining the main methodology used in the study.
To determine whether TLE6 deficiency led to erratic mating behaviour, the team mated both TLE6-deficient and wild-type male mice with wild-type females. Interestingly, mating frequency and the number of offspring did not differ between the two groups. Furthermore, embryos derived from TLE6-deficient male mice showed similar developmental rates as those from wild-type mice.
However, when investigating why the traits associated with TLE6 deficiency were not passed on to the next generation, the researchers shifted their focus to sperm function. Kazama said, “We hypothesised that the difficulty in transmitting genetic traits from TLE6-deficient male mice could be due to reduced sperm count and motility.” Indeed, their analysis of the testes and sperm revealed a significant reduction in sperm count and a marked decrease in sperm motility. Immunofluorescence staining revealed that the TLE6 protein in deficient mice was localised in the sperm’s midpiece, a region associated with mitochondria – critical for energy production. This suggests that TLE6 may play a role in energy production necessary for sperm motility. Gene expression analysis further confirmed an overall increase in genes related to sperm structure, motility, and fertilisation in the testes of TLE6-deficient mice.
“The role of TLE6 in the development of sperm cells may vary between humans and mice. Therefore, further research is necessary to clarify the mechanisms by which TLE6 deficiency causes sperm abnormalities in TLE6 hetero knockout mice and to explore its clinical relevance in humans,” concludes Kazama.
This study provides new insights into the genetic causes of male infertility and highlights the potential for future research and development of new assisted reproductive technologies. By understanding the role of TLE6, this work may pave the way for novel treatments that address male infertility at the genetic level.
This study was published in Frontiers in Cell and Developmental Biology.
Related topics
Animal Models, CRISPR, Gene Therapy, Genetic Analysis, Genome Editing
Related conditions
infertility
Related organisations
Kanazawa Medical University
Related people
Hirofumi Nishizono, Kousuke Kazama, Yuki Miyagoshi