UC Davis researchers achieve total synthesis of ibogaine
Posted: 10 February 2025 | Drug Target Review | No comments yet
UC Davis researchers achieve a breakthrough in synthesising ibogaine, enabling safer drug development for addiction and depression treatment.

Researchers at the University of California, Davis Institute for Psychedelics and Neurotherapeutics (IPN) have achieved a breakthrough in the total synthesis of ibogaine, a psychoactive plant derivative with potential therapeutic applications. This discovery opens doors for further research into the properties of ibogaine and related compounds, potentially leading to safer and more effective medicines. The findings were published in the journal Nature Chemistry.
Efficient synthesis from readily available chemicals
Ibogaine, extracted from plants native to Africa, has shown promise in treating addiction and depression. However, its limited natural availability and potential cardiac risks have hindered its widespread use and research. The UC Davis team have overcome this challenge by synthesising ibogaine, ibogaine analogues, and related compounds from pyridine, a relatively inexpensive and widely available chemical.
Ibogaine’s complex chemical structure makes it hard to produce in significant quantities, and this challenging chemistry has historically limited medicinal chemistry efforts to develop improved analogues.
“Ibogaine’s complex chemical structure makes it hard to produce in significant quantities, and this challenging chemistry has historically limited medicinal chemistry efforts to develop improved analogues,” explained David E. Olson, director of the IPN and a professor of chemistry and biochemistry and molecular medicine at UC Davis. “Performing total synthesis solves both problems. We can make it without having to harvest tons and tons of plant material and we can also make analogues, several of which are demonstrating really interesting properties.”
The team’s synthetic strategy enabled the creation of four naturally occurring ibogaine-related alkaloids and several non-natural analogues. Impressively, overall yields ranged from 6 percent to 29 percent after only six or seven steps, a significant improvement in efficiency compared to previous attempts.
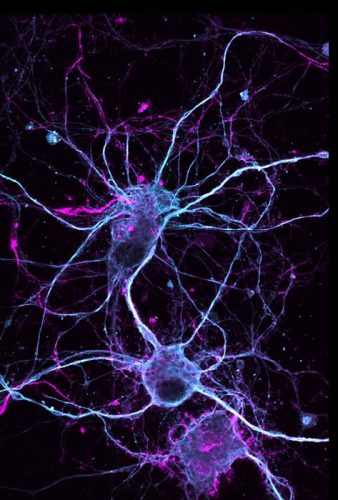

In a study appearing in Nature Chemistry, UC Davis researchers report the successful total synthesis of ibogaine, ibogaine analogues and related compounds from pyridine. Here we see a neuron treated with the ibogaine analogue (-)-10-fluoroibogamine, which showcased effects on neuronal growth and connection. Credit: Andy Domokos/UC Davis
Therapeutic potential of ibogaine analogues
Despite the cardiac risks associated with ibogaine, its popularity as a treatment for substance use disorders, traumatic brain injury, and other conditions is growing. Olson acknowledged this, stating, “Some people want to find ways to administer ibogaine more safely and you might be able to mitigate risk with careful cardiac monitoring and magnesium supplementation. But maybe we just need ibogaine 2.0, a better version that still produces these profound anti-addictive and anti-depressant effects but doesn’t have that cardiac risk.”
Maybe we just need ibogaine 2.0, a better version that still produces these profound anti-addictive and anti-depressant effects but doesn’t have that cardiac risk.
The study highlighted two particularly interesting ibogaine analogues. The first was the mirror image of ibogaine, a concept known as chirality in chemistry. Researchers found that only the naturally occurring version promoted neuronal growth. “This allowed us to show for the first time that ibogaine’s effects are probably the result of it being bound to a particular receptor,” Olson explained. “We don’t have all the details of what receptor that is, but the unnatural compound is a good tool for probing this biology.”
The second analogue of interest was (-)-10-fluoroibogamine. This compound demonstrated exceptional effects on neuronal structure and function, promoting both growth and reconnection. It also showed powerful effects on serotonin transporters, proteins that regulate serotonin levels in synapses. “The serotonin transporter is the target of many antidepressants and is hypothesized to be relevant to ibogaine’s therapeutic efficacy,” Olson said. These findings suggest that (-)-10-fluoroibogamine warrants further investigation as a potential treatment for substance use disorders, depression, and related neuropsychiatric diseases.
A roadmap for future research
This research represents a decade-long effort, with the team exploring various synthetic routes to achieve this breakthrough. “A lot of these iboga alkaloids and ibogaine analogues are not made from cheap, readily available starting materials,” Olson noted. “The difference with our strategy is that we rely on very abundant, inexpensive chemicals, and we can assemble the pieces in just a few steps. Overall, our goal was to create a more efficient process.”
The researchers hope that their total synthesis strategy will provide a valuable roadmap for other scientists, enabling them to efficiently access ibogaine analogues and ultimately develop safer and more effective medicines for a range of neuropsychiatric conditions.
The research was supported by grants from the National Institute of General Medical Sciences, the National Institute on Drug Abuse, and the Camille Dreyfus Teacher-Scholar Award.
Related topics
Drug Discovery, Drug Discovery Processes, Neuroprotection
Related conditions
Addiction, Depression, Neuropsychiatric disorders
Related organisations
Institute for Psychedelics and Neurotherapeutics (IPN), University of California-Davis (UC Davis)
Related people
David E. Olson








