Novel radioactive drug targets aggressive metastatic melanoma
Posted: 25 March 2025 | Drug Target Review | No comments yet
Scientists in Japan have developed a new radioactive drug that emits alpha particles, showing promise for targeting metastatic melanoma – an aggressive skin cancer resistant to many conventional treatments.

Metastatic melanoma is the most aggressive form of skin cancer, known for its rapid spread to other organs and its resistance to conventional treatments. While therapies such as immunotherapy and targeted drugs have provided some success, they remain limited in effectiveness. In a significant breakthrough, researchers from Japan have developed a novel radioactive drug that emits alpha particles, offering a promising new avenue for targeted treatment.
The need for advanced radiotherapy
Development of a novel 211At-labeled peptide drug
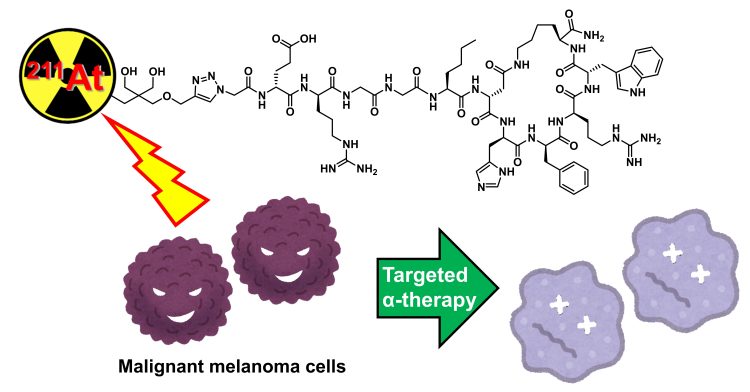
To improve the precision of radiotherapy, the research team developed an astatine-211 (211At)-labelled peptide drug. This innovative treatment approach resulted from a collaborative effort between Assistant Professor Hiroyuki Suzuki of Chiba University, Dr Noriko S. Ishioka of the National Institutes for Quantum Science and Technology, Dr Hiroshi Tanaka of Juntendo University, and Dr Tadashi Watabe of Osaka University. Their findings were published in the European Journal of Nuclear Medicine and Molecular Imaging.
TAT relies on radioisotopes that emit alpha particles, which have a short emission range but deliver high-energy radiation
TAT relies on radioisotopes that emit alpha particles, which have a short emission range but deliver high-energy radiation. This characteristic allows for precise targeting of tumour cells, minimising damage to the surrounding healthy tissues. To do this, the researchers designed an astatine-211-labeled α-melanocyte-stimulating hormone (α-MSH) peptide analogue called [211At]NpG-GGN4c. This drug specifically targets melanocortin-1 receptors (MC1R), which are overexpressed in melanoma cells, ensuring high tumour selectivity and reduced radiation exposure to non-target areas.

Metastatic melanoma is the most aggressive form of skin cancer, often spreading rapidly to other organs and resisting conventional treatments, meaning advanced therapies are crucial for improving outcomes.
Promising preclinical results
The efficacy of the newly developed drug was tested on B16F10 melanoma-bearing mice models. The researchers conducted biodistribution analyses to assess tumour uptake, organ clearance, and overall stability of the compound. Dr Tomoya Uehara from Chiba University explained the methodology, stating: “We treated the mice with different doses of the compound while monitoring the tumour response, body weight, and survival rates over time. We found a dose-dependent inhibitory effect in a melanoma-bearing mouse model, confirming the effectiveness of our approach.”
The drug exhibited high accumulation in tumours while demonstrating rapid clearance from non-target organs, confirming its specificity for MC1R on melanoma cells. Additionally, tumour growth was significantly suppressed in a dose-dependent manner, and the drug showed high stability in blood plasma, minimising the risk of radioactive leakage in the body.
Future implications and clinical prospects
The success of [211At]NpG-GGN4c in preclinical trials points to a promising future for alpha-emitting radiopharmaceuticals in cancer therapy. Dr Suzuki emphasised that the molecular design of their synthesised drug could be applied to other 211At-labeled radiopharmaceuticals, potentially broadening its impact beyond melanoma. “We believe our approach could open up new possibilities for treating refractory cancers beyond melanoma.” He continued: “If successfully translated into human trials, this therapy may emerge as a viable treatment option for patients with advanced melanoma in the coming years, and could provide new therapeutic opportunities for patients with refractory cancer.”
The development of this new radioactive drug marks a significant milestone in the fight against metastatic melanoma, offering hope for a more effective and targeted treatment option in the years to come.
This study was published in the European Journal of Nuclear Medicine and Molecular Imaging.
Related topics
Cancer research, Clinical Trials, Immuno-oncology, Immunotherapy, Oncology, Radiotherapy
Related conditions
Metastatic melanoma, Skin cancer