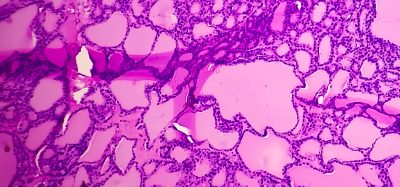
Key PKD findings could accelerate development of new treatments
Posted: 16 April 2025 | Drug Target Review | No comments yet
Rutgers researchers have discovered new insights into how polycystic kidney disease (PKD) progresses, which could lead to more targeted treatments. This breakthrough may help improve therapies for PKD patients in the future.

Polycystic kidney disease (PKD) is a genetic disorder that severely impacts the kidneys, causing cysts to form and impairing the organ’s ability to remove waste from the body. Affecting over 12.4 million people worldwide, the dominant form of the disease is progressive and often leads to kidney failure, requiring treatments such as dialysis or transplantation. While these methods help manage the symptoms, they do not cure the disease. However, new research from Rutgers University offers promising new insights that could eventually lead to more effective therapies.
Tracking the progression of PKD through extracellular vesicles
In a new study published in Nature Communications, a team of Rutgers’ geneticists led by Inna Nikonorova, a research assistant professor in the Department of Genetics, has made a significant discovery regarding how PKD progresses at the molecular level. Nikonorova’s work focuses on extracellular vesicles (EVs), which are tiny, sub-microscopic particles that are shed by cells and serve as communication tools within the body. These vesicles have been recognised for their role in the development of various diseases, including cancers, neurodegenerative conditions, and kidney diseases like PKD.
Extracellular vesicles were once considered to be mere waste products of cells, but researchers have since realised that they play a much more complex role in cellular health and disease. These vesicles can carry beneficial cargo, such as proteins that aid in tissue regeneration and wound healing. On the other hand, they can also transport harmful proteins that act as disease mediators, spreading toxicity throughout the body. The key question that had remained unanswered was how cells decide which proteins and materials to package and send inside these vesicles.
A novel method for tracking EV cargo
To solve this mystery, Nikonorova and her co-researcher, Maureen Barr, distinguished professor of genetics at Rutgers University-New Brunswick, focused on a specific EV that transports PKD-related proteins called polycystins. Changes in these polycystins are strongly linked to the progression of PKD, making them an essential target for research.
Using innovative techniques, Nikonorova developed a labelling tool that allowed her to track the movement of these specialised EVs. She applied this tool in a laboratory worm, C. elegans, known for its translucent body and rapid growth cycle. By using a green fluorescent protein that specifically binds to polycystin-2, a crucial protein in PKD, Nikonorova was able to observe the movement of EVs carrying polycystins through the worm’s body.
This method of tracking, known as ‘proximity labelling,’ allowed Nikonorova to map out the interactions between polycystins and the other proteins they travel with. She described the technique as like giving someone a flashlight and watching them move room to room through a dark house, shedding light on the path of the polycystins as they moved through the body.
Beyond identification: understanding protein interactions
Nikonorova’s approach goes beyond simply identifying the proteins carried by EVs. While previous studies have catalogued the proteins present within these vesicles, her research aimed to understand the intricate relationships between these proteins and how they interact with polycystins. Nikonorova and her team looked at whether the identified proteins traveled with polycystins within EVs and how they interacted with each other.
The research reveals a complex network of interactions that could provide crucial insights into how PKD progresses. By understanding these interactions and how polycystins are packaged into EVs, scientists can begin to uncover the molecular mechanisms behind the disease. This information is critical for designing potential therapies to either replace missing polycystin proteins or slow the disease’s progression.
A step closer to targeted therapies for PKD
Nikonorova’s findings offer a promising new direction for PKD research. By tracking the cargo within EVs and understanding the proteins involved in PKD, this research provides essential knowledge about what happens at the cellular level in patients with the disease. This could lead to new therapeutic strategies aimed at restoring or mimicking the function of polycystins, providing hope for better treatments for PKD patients in the future.
The study was supported by grants from the National Institutes of Health, highlighting the importance of ongoing research and funding in the pursuit of new treatments for PKD and other genetic diseases.
Related topics
Disease Research, Drug Discovery, Drug Discovery Processes, Genetic Analysis, Genomics, Translational Science
Related conditions
polycystic kidney disease (PKD)
Related organisations
National Institutes of Health, Rutgers University
Related people
Inna Nikonorova, Maureen Barr