Reprogramming gut cells to treat short bowel syndrome
Posted: 18 April 2025 | Drug Target Review | No comments yet
A preclinical study conducted by researchers at Weill Cornell Medicine have demonstrated a new gene-editing strategy to treat short bowel syndrome (SBS), a life-threatening condition in which patients lack a functional small intestine.

Researchers from Weill Cornell Medicine have demonstrated a promising new approach to treating short bowel syndrome (SBS), a life-threatening condition in which patients have a significantly reduced small intestine due to surgery or disease. By knocking out a single gene, they successfully reprogrammed part of the large intestine to function like the small intestine, providing hope for new therapeutic options for those suffering from this debilitating disorder.
Short bowel syndrome and its challenges
Short bowel syndrome typically arises after extensive surgery to remove portions of the small intestine due to chronic conditions such as cancer, trauma, or inflammatory diseases. The small intestine is essential for nutrient absorption, and without it, patients are often forced to rely on intravenous nutrition to survive. However, despite this life-saving intervention, many SBS patients face significant challenges in terms of health and quality of life.
The breakthrough discovery: SATB2 gene knockout
In a new study published in Gastroenterology, Dr Xiaofeng Steve Huang and his team investigated the potential of manipulating the SATB2 gene in preclinical models of SBS. SATB2 is a gene previously shown to play a crucial role in maintaining the identity of colon cells. When deleted in the colon, SATB2 prompts cells to adopt characteristics of the ileum, the lower portion of the small intestine.
The team’s discovery came when they applied this gene-editing strategy to a preclinical model of short bowel syndrome. By knocking out SATB2, they found that cells in the upper colon reprogrammed to function similarly to small intestine cells. This change restored nutrient absorption and even reversed weight loss in the treated mice, suggesting that the gene-editing strategy could be a game-changer for SBS patients.
A step toward gene therapy for SBS
The results of the study were remarkable. Mice treated with SATB2 deletion were able to recover their normal body weight and demonstrated a significantly improved survival rate compared to control mice. Four out of five treated mice survived for over 60 days, whereas only 10 percent of control mice survived that long.
“The results were striking,” said Huang, senior author of the study. “By reprogramming the upper colon to take on the functions of the small intestine, we were able to restore nutrient absorption and improve survival in a model of short bowel syndrome.”
The tissue structures in the treated mice resembled those found in the ileum, including the presence of blood and lymph vessels, which are essential for proper nutrient absorption. These findings suggest that the approach could one day lead to a gene therapy for SBS, offering a new hope for patients who currently rely on intravenous nutrition.
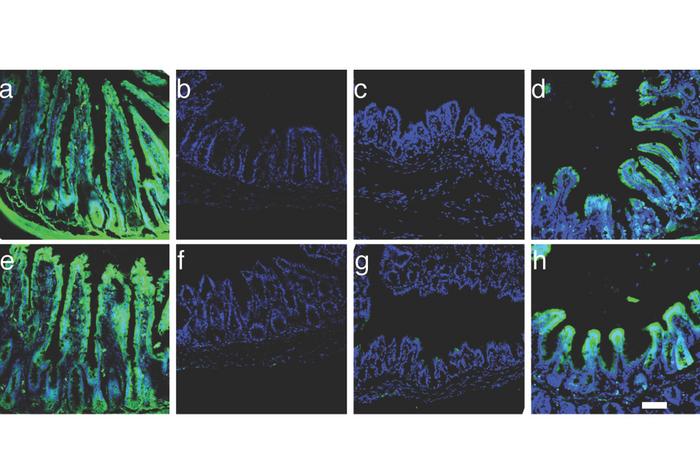

SATB2 deficiency confers small intestinal (ileal) properties on colon cells. Green fluorescent markers bound to cholesterol (top row) and glucose (bottom row) show nutrient absorption in: a) Ileal tissue; b) Colon tissue; c) Colon tissue in mice with most of the small bowel removed; d) Colon tissue in mice with most of the small bowel removed and SATB2 knocked out. Credit: Dr Tao Liu.
Organoids: a step closer to human applications
Taking the research one step further, the team tested their strategy on human colon-derived organoids. These small, organ-like tissue clumps were genetically modified using an adenovirus-associated virus (AAV) to delete the SATB2 gene. The treated organoids developed characteristics of the ileum, and when transplanted into mice, they survived and functioned similarly to small intestine tissue.
This success in human-derived tissue brings the team closer to developing a gene therapy for SBS. Manipulating colon cells in this way could lead to more effective treatments and potentially a cure for this challenging condition.
Looking ahead: testing in more advanced models
While the results are promising, Dr Huang and his team are not stopping here. They plan to continue testing their approach in more advanced preclinical models of SBS, including larger animals and eventually human clinical trials. The researchers are also exploring ways to refine the technique and enhance its effectiveness in clinical settings.
“We’re excited about the potential of this research to transform how we treat short bowel syndrome,” said Huang. “Our goal is to develop a gene therapy that can restore normal function in the intestine and help patients live healthier lives without the need for lifelong intravenous nutrition.”
Conclusion
The study represents a significant step forward in the fight against short bowel syndrome, which affects thousands of people around the world. By reprogramming the large intestine to take on the functions of the small intestine, Weill Cornell Medicine investigators have made huge strides towards potential treatments for SBS and other gastrointestinal disorders. As the team continues to develop and refine their approach, the hope for a future gene therapy for SBS becomes more achievable than ever before.
Related topics
Animal Models, Disease Research, Drug Development, Gene Therapy, Molecular Biology, Organoids, Regenerative Medicine, Therapeutics, Translational Science
Related conditions
short bowel syndrome
Related organisations
Weill Cornell Medicine
Related people
Dr Xiaofeng Steve Huang