Bacteria-boosted nanoparticles offer a triple threat against cancer
Posted: 15 April 2025 | Drug Target Review | No comments yet
A new cancer therapy combines multiple treatment strategies into a single graphene oxide-based nanocomposite. It uses bacterial components to enhance immune response and scalability, creating a powerful and cost-effective approach to tackling tumours.

Modern cancer treatment is rapidly evolving beyond conventional chemotherapy. A growing array of targeted therapies, including immunotherapy, radiation therapy, and photothermal therapy, is reshaping the way we fight this complex disease. Now, researchers in Japan have developed an innovative nanocomposite, enhanced with bacteria, that combines all three approaches into one powerful treatment strategy
In a study led by Professor Eijiro Miyako at the Japan Advanced Institute of Science and Technology (JAIST), has introduced a graphene oxide (GO)-based nanocomposite. This nanocomposite is designed to deliver chemotherapy, trigger immune responses, and destroy tumours through photothermal therapy. The team’s findings were published online on March 21, 2025, and will appear in Carbon on May 5, 2025.
Graphene oxide with a microbial makeover
Graphene oxide is known for its exceptional properties, high surface area, biocompatibility, and efficient photothermal conversion, making it a promising candidate for targeted cancer therapy. However, its clinical application has been hindered by challenges in dispersibility and scalability.
To solve this, Miyako’s team turned to bacteria. Certain bacteria naturally enhance immune responses and improve material dispersion due to their amphiphilic membrane components. Leveraging these traits, the team incorporated bacterial elements from Cutibacterium acnes (CA) into their nanocomposite design.
Certain bacteria naturally enhance immune responses and improve material dispersion due to their amphiphilic membrane components.
The result: a multifunctional nanoparticle consisting of GO, CA components, and the chemotherapeutic agent camptothecin (CPT). This nanocomposite attacks tumours on three fronts: CPT delivers localised chemotherapy, GO enables heat-induced tumour destruction, and CA elements activate the body’s immune defences.
“CPT delivers localised chemotherapy, GO intensifies heat-based tumour destruction, and CA components activate immune defences; together, these effects provide a highly promising cancer treatment,” says Miyako.
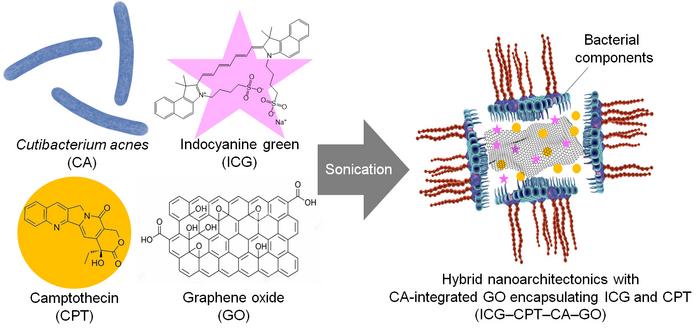

Schematic of the modified graphene oxide nanoparticle (right) illustrates the integration of a chemotherapy drug camptothecin (orange circle), a fluorescent dye (pink star), and bacterial coating of Cutibacterium acnes onto graphene oxide nanoparticle. Credit: Eijiro Miyako from JAIST
Triple-action therapy in a single particle
To create the nanocomposites, researchers sonicated GO, CA components, and CPT in a cell culture medium. The resulting particles, approximately 53 nanometres in size, were stabilised with a bacterial coating that improved their dispersibility and biocompatibility.
In mouse models of colorectal cancer, the nanoparticles demonstrated remarkable tumour-targeting abilities. Leveraging the enhanced permeability and retention (EPR) effect, they accumulated at tumour sites while sparing healthy tissue. Even without laser activation, the combination of immune stimulation and chemotherapy significantly suppressed tumour growth.
To amplify the treatment effect, a low-power laser (0.8 W for five minutes) was applied, raising tumour temperatures to 50 degrees, which is sufficient enough to induce cancer cell death without harming surrounding tissues. After five laser treatments, the mice experienced complete tumour eradication and full recovery.
Immune activation and a scalable future
Further analysis revealed strong immune activation, with increased presence of T cells, B cells, neutrophils, macrophages, and natural killer cells, which are all driven by the CA bacterial components. This multi-layered immune response adds a vital dimension to the nanocomposite’s therapeutic potential.
This multi-layered immune response adds a vital dimension to the nanocomposite’s therapeutic potential.
What sets this approach apart from conventional GO modifications is its simplicity. Traditional chemical modifications often require costly reagents and complex steps. By contrast, the JAIST team’s method uses inexpensive bacterial cultures and a single-step sonication process, making it both cost-effective and scalable.
“Cancer is a highly progressive and complex disease, requiring a multidimensional approach to fight it,” Miyako notes. “The proposed approach is cost-effective, requires minimal resources, and is easily scalable for mass production.”
Toward next-generation cancer therapies
This study marks a new era in nanomedicine, where naturally derived materials and multifunctional platforms could transform cancer treatment. By combining immune activation, targeted drug delivery, and photothermal effects into one nanoparticle, the JAIST team has developed a strategy that not only effectively targets tumours but also makes the treatment more accessible and practical for real-world use.
Related topics
Cancer research, Drug Delivery, Immunotherapy, nanohyperthermia, Nanomedicine, Nanoparticles, Translational Science
Related conditions
Cancer
Related organisations
Japan Advanced Institute of Science and Technology (JAIST)
Related people
Professor Eijiro Miyako