Scientists uncover steps to kinase enzyme activation
Posted: 18 May 2018 | Drug Target Review | No comments yet
A new study by the University of Arizona (UA) suggests that revealing all the steps required to activate an enzyme called a protein kinase may help identify new ways to target cancer.
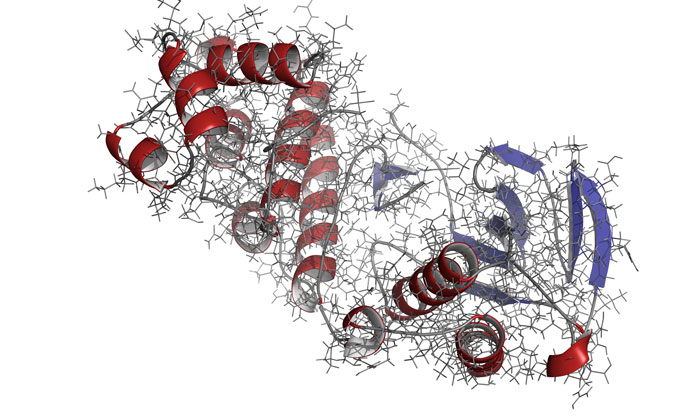

The scientists identified that not only must the kinase have all necessary parts in place, but the enzyme must move in the correct manner and at the correct speed in order to function properly in a cell.
First author Senthil Kumar Ganesan, a UA research assistant professor of chemistry and biochemistry, explained that kinases are switches that turn certain cellular processes on or off. If a cell’s kinases stopped working, the cell would die.
The UA-led team is the first group to determine the full pathway for activating a protein kinase, said senior author Wolfgang Peti, who holds the Homer C. and Emily Davis Weed Endowed Chair in Chemistry at the University of Arizona.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
Commenting on the value of their findings, Peti said: “Kinases are one of the major cancer drug targets. If you know how a kinase works, you can definitely better design your drugs against it.”
However, developing a drug that takes advantage of this new research finding is likely to take at least 10 years, he said.
To observe a kinase called mitogen-activated protein kinase (MAPK) p38 in action, the researchers used nuclear magnetic resonance (NMR) spectroscopy.
Molecules bind for kinase activation
All necessary molecules had to bind to p38 for the enzyme to become active. Only then could the enzyme move correctly to do its job in the cell, noted the researchers.
“We figured out how it moves to do its job and what the contribution of speed of movement is to its job,” Peti said.
The finding suggests a new avenue for developing anti-cancer drugs could be creating drugs that interfere with p38’s movement, he said. “We are excited because this kinase belongs to a family of kinases that are called MAP kinases – key signalling kinases that regulate a plethora of pathways (within cells),” he said.
Peti commented that the new finding might apply to other kinases, too, given that many kinase enzymes in the body are very similar and are therefore likely to have similar paths to activation.
The National Institutes of Health grant NIH-R01GM100910 funded the research and the data was collected at the UA Molecular Structures Core and Brown University Structural Biology Core facilities. An oft-used analogy for how enzymes work is that of a lock and a key. A particular molecule, the key, fits into a specific site in the enzyme, the lock. Once the key is in the lock, the enzyme can start working. The key-in-the-lock is necessary for an enzyme to work, but activating an enzyme isn’t always so simple. Some scientists have suggested that dynamics – the manner and speed at which an enzyme moves – is important for activating kinase enzymes.
Peti is interested in how molecules such as kinases govern processes within cells, so he wanted to investigate the role that dynamics plays. Learning more about how molecules function inside cells can help develop targeted drugs to combat cancer and other diseases.
Ascertaining the role that dynamics plays in the activation of p38 was more difficult and took longer than he expected. This is because the tool the scientists used to monitor the enzyme in action, NMR spectroscopy, is more complicated with a large molecule like p38. In addition, the team had to develop new computer software to analyse the results, Peti explained.
The researchers observed the enzyme over a series of steps as various key molecules bound to p38. Some enzymes become activated as key molecules bind – but that didn’t happened in this case.
“If you think it’s a five- or six-step process to go from zero to 100, we thought that at steps zero, one, two or three, we would get indications or guidelines,” Peti said. When that didn’t happen, he said that the researchers considered giving up.
“Nothing was working, and it looked as if our hypothesis was completely wrong,” he said.
“What we got is nothing happening from zero to one, there’s nothing happening from one to two, there’s nothing happening from two to three, there’s nothing happening from three to four,” Peti said. “When you go from four to five: Boom! Suddenly it was there.”
Once all those steps had been completed, although the shape of p38 didn’t change its dynamics did. Peti explained that final change in dynamics was required for the enzyme to work.
The team’s next step is investigating whether the motion of kinases plays a role in diabetes.
The study was published in Proceedings of the National Academy of Sciences.
Related topics
Analysis, Enzymes, kinase enzyme, Nuclear Magnetic Resonance (NMR)
Related conditions
Cancer
Related organisations
National Institutes of Health (NIH), University of Arizona
Related people
Senthil Kumar Ganesan, Wolfgang Peti