First in vitro lung cancer test launched
Posted: 12 June 2019 | Victoria Rees (Drug Target Review) | No comments yet
A new in vitro lung cancer diagnostic test has been launched by Roche Diagnostics to provide improved detection of cancer.
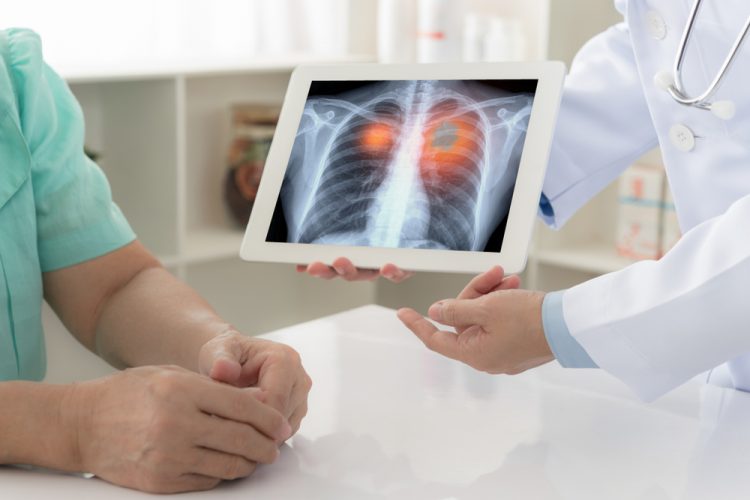

The first in vitro diagnostic immunohistochemistry (IHC) assay to provide a cost-effective and efficient way to initially detect elevated ROS1 protein expression in cancer has been launched. The VENTANA ROS1 (SP384) Rabbit Monoclonal Primary Antibody may also be applied in identification of ROS1-positive lung cancer.
Mutations of the ROS1 gene are present in some non-small cell lung cancer (NSCLC). These can help to discover which patients would respond to targeted treatment.
“Our highly sensitive ROS1 test is the first in vitro diagnostic test available for recommended lung cancer testing guidelines, with the added benefit of rapid turnaround time,” said Pierre Hazlewood, marketing director at Roche Diagnostics, who launched the test.
These types of cancer are rare and only found in up to 2 percent of NSCLC cases. This means the treatment will provide a cost-effective and efficient way to pinpoint cases of elevated ROS1 protein expression. This can indicate which method to use next, such as fluorescence in situ hybridisation (FISH) or next generation sequencing (NGS).
Guidelines from the College of American Pathologists and the National Comprehensive Cancer Network, US, recommend ROS1 testing for confirmed lung adenocarcinoma cases.
Related topics
Assays, Drug Targets, In Vitro, Screening
Related conditions
Cancer, non-small cell lung cancer
Related organisations
College of American Pathologists, National Comprehensive Cancer Network, Roche Diagnostics
Related people
Pierre Hazlewood