Structure of crucial metabolic enzyme revealed by high-end microscopy
Posted: 29 August 2019 | Rachael Harper (Drug Target Review) | No comments yet
Scientists reveal the atomic structure and regulative mechanism of the metabolic enzyme transhydrogenase.
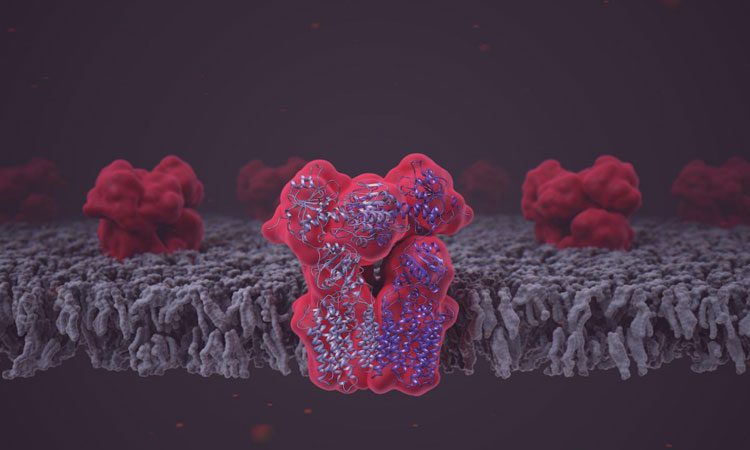

The enzyme transhydrogenase (NNT) embedded into the lipid bilayer of the mitochondrial membrane (credit: Martina Maritan).
Researchers have used high-end microscopy to reveal the structure and function of a crucial metabolic enzyme. The authors of the study say their findings could be used in the development of novel therapies for metabolic dysfunctions including metabolic syndrome and some cancers.
Balancing the two opposing processes of breaking down molecules derived from food to generate energy and producing new molecules is accomplished by an enzyme called proton-translocating transhydrogenase or NNT (nicotinamide nucleotide transhydrogenase). NNT sits in the mitochondria’s membrane and uses the electrochemical proton gradient generated by cellular respiration to provide the mitochondria with the right amount of the co-enzyme NADPH, a vital metabolic precursor.
However, the details of how NNT accomplishes the coordinated transfer of protons across the membrane and synthesis of NADPH have remained obscure due to the lack of knowledge about the enzyme’s atomic structure.
Domen Kampjut, PhD student at IST Austria, and his supervisor and group leader Professor Leonid Sazanov, have for the first time visualised the molecule of mammalian NNT at a scale that allowed them to identify the structural principles of the enzyme’s channel gating and gain a deeper understanding of its functioning and malfunctioning.
The atomic analysis of the enzyme NNT was only possible with developments in cryo-electron microscopy (cryo-EM). Parts of the current study’s data were generated using a new cryo-electron microscope.
The cryo-EM analysis of NNT delivered near atomic-resolution images of the molecule’s three different domains in various conformational states.
The images showed how the domain that binds NADPH can open the proton channel to either side of the mitochondrial membrane.
“These structures are particularly exciting because transhydrogenase performs an amazing volte-face by rotating an entire, quite large, NADPH-binding domain 180 degrees ‘up’ or ‘down’,” said Professor Leonid Sazanov. “This is, as far as we know, unique among studied enzyme mechanisms. However, such a rotation now makes complete sense in view of our proposed mechanism and it shows how nature can ‘creatively’ solve challenging tasks.”
The results are an important further step towards the development of novel therapies. For instance, the development of currently unavailable NNT inhibitors has great therapeutic potential with regard to metabolic dysfunctions.
Related topics
Enzymes, Imaging, Microscopy, Protein, Research & Development, Screening
Related conditions
Cancer, Metabolic syndrome
Related organisations
IST Austria
Related people
Domen Kampjut PhD, Professor Leonid Sazanov