Study reveals neuronal role in glioblastoma growth and how to prevent it
Posted: 20 September 2019 | Victoria Rees (Drug Target Review) | 1 comment
Researchers have discovered neurons send electrical signals to glioblastoma tumours, causing them to grow but have also identified methods of prevention in models.
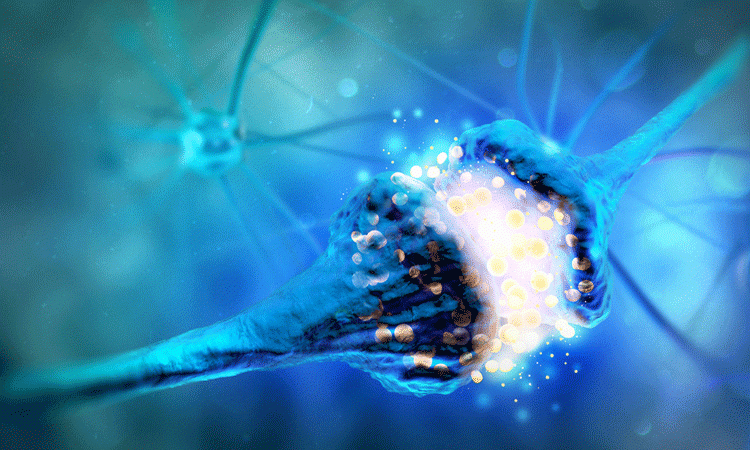

Researchers have discovered how neurons in the brain establish contact with aggressive glioblastomas, which promotes tumour growth. Also revealing a way to slow tumour growth, they say their findings could be used in the development of anti-cancer therapies.
A team from Heidelberg University Hospital and the German Cancer Research Center (DKFZ), both Germany, found that neurons can form a synapse-like relationship with tumour cells of glioblastomas. The tumour then benefits from this signalling, which the researchers conclude drives tumour growth.
“The tumour cells are not only interconnected in the brain like neurons; they also receive direct signals from them,” explained Frank Winkler, whose research group is affiliated with the University Hospital and the DKFZ.
How the activation of the tumour leads to increased tumour growth is yet to be discovered, but the researchers were able to block the process in the animal models”
The researchers used mice models carrying human glioblastoma tissue, cell cultures with human neurons and tumour cells, as well as tissue samples from patients.
They used a wide range of modern microscopy methods to provide three-dimensional (3D) images of the connections between neurons and tumour cells. Electrical recordings from the tumours revealed currents generating from the synaptic connections, mediated by AMPA subtype glutamate receptors.
How the activation of the tumour leads to increased tumour growth is yet to be discovered, but the researchers were able to block the process in the animal models.
The methods include a significant reduction of brain activity (for example anaesthetic), pharmacological interventions that interrupt binding of the neurotransmitters on the AMPA receptor or blocking the AMPA receptor using genetic engineering. In all of these cases, tumour spread became slower in animal experiments.
“This project began with an observation in basic research. In close cooperation with our clinical partners, it has led to conceptually new insights which will allow new treatment approaches to be developed using targeted translational research,” said Thomas Kuner, Director of the Department of Functional Neuroanatomy at the Institute for Anatomy and Cell Biology.
The results were published in Nature.
Related topics
Drug Targets, Neurons, Oncology, Research & Development
Related conditions
Glioblastoma
Related organisations
German Cancer Research Center (DKFZ), Heidelberg University Hospital
Related people
Frank Winkler, Thomas Kuner








Good morning,
Perineuronal satellitosis, the microanatomical clustering of glioma cells around neurons in the tumor microenvironment, has been recognized as a histopathological hallmark of high-grade gliomas since the seminal observations of Scherer in the 1930s.
neuron‒glioma cell interactions regulate malignancy and that neuronal activity is a critical determinant of glioma growth and progression. Elucidation of the interplay between normal and malignant neural circuitry is critical to realizing the promise of effective therapies for these seemingly intractable diseases.