Accurate model of organ scarring created using stem cells in a lab
Posted: 16 December 2019 | Rachael Harper (Drug Target Review) | No comments yet
Researchers have developed a ‘scar in a dish’ model derived from human stem cells which mimics the progressive scarring that occurs in human organs and has lead to a drug candidate being identified to stop the progression of fibrosis.

A team of researchers from the University of California, Los Angeles, US have developed a ‘scar in a dish’ model that uses multiple types of cells derived from human stem cells. The model closely mimics the progressive scarring that occurs in human organs and it has been used to identify a drug candidate that stopped the progression of and even reversed fibrosis in animal models.
“Millions of people living with fibrosis have very limited treatment options,” said Dr Brigitte Gomperts, a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, who led the study. “Once scarring gets out of control, we don’t have any treatments that can stop it, except for whole-organ transplant.”
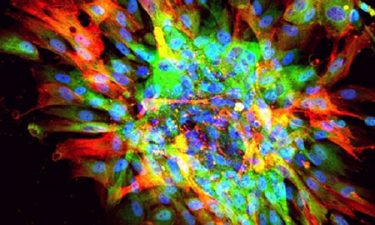

Microscopic image of scarring in a dish, showing evidence of fibrosis (red, green) and nuclei of all cells (blue) (credit: UCLA).
The model was created using induced pluripotent stem cells, or iPS cells. Traditionally, when iPS cells are used to model scarring and other disorders, they are first coaxed to produce one specific cell type through a process called directed differentiation. To generate their model, the team took a different approach and let their iPS cells do what stem cells would normally do in the human body, which is to produce a range of cell types.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
“Fibrosis likely occurs as the result of interactions between multiple different cell types, so we didn’t think it made sense to use just one cell type to generate a scarring model,” said Preethi Vijayaraj, the study’s first author and an assistant adjunct professor of paediatric hematology/oncology at the David Geffen School of Medicine at UCLA and a member of the UCLA Jonsson Comprehensive Cancer Center.
The resulting mixture contained many types of cells that are thought to play a role in fibrosis, including mesenchymal cells, epithelial cells and immune cells. All of the cells in the mixture retained a degree of plasticity, meaning they had the capacity to change cell types.
Gomperts’ model is the first to recreate that plasticity, a hallmark of progressive fibrosis.
To bring about the scarring process, the team placed the cell mixture on a rigid hydrogel that recreated the approximate stiffness of a scarred organ. The cells responded in the same way that they respond to injury in the body, by producing signals of injury or damage and activating a molecule called transforming growth factor beta, or TGF beta, which serves as the spark that sets off fibrosis.
After confirming that the new model accurately recreated fibrosis in human organs, the team set out to identify drugs that could halt or reverse the process and identified a small molecule that stopped progressive scarring and healed the damage it had caused. The researchers suspect the molecule works by activating the cells’ inherent wound healing processes.
“This drug candidate seems to be able to stop and reverse progressive scarring in a dish by actually breaking down the scar tissue,” said Gomperts. “We tested it in animal models of fibrosis of the lungs and eyes and found that it has promise to treat both of those diseases.”
Moving forward, the team plans to pin down how that drug candidate reverses scarring and screen additional small molecules to gain a better understanding of the disease and identify more drug candidates.
Their study was published in Cell Reports.
Related topics
Drug Discovery, Drug Targets, Induced Pluripotent Stem Cells (iPSCs), Research & Development, Small Molecules, Stem Cells
Related conditions
fibrosis
Related organisations
University of California Los Angeles (UCLA)
Related people
Dr Brigitte Gomperts, Preethi Vijayaraj